A paper written
by S. Nassir Ghaemi, MD was posted this week and in it he discussed the concept
of diseases modifying medications and whether any medication used for
psychiatric purposes might be included in that category. Dr. Ghaemi is a distinguished psychiatrist
who has written on diverse topics. He is
a prominent psychiatric theorist and also has complied many of his ideas about
psychiatry and psychopharmacology in the book Clinical Psychopharmacology (1).
In the book he
presents a brief discussion of disease modifying medications and how few there
seem to be in psychiatry as well as what he considers to be obstacles to the
discovery of these agents. He does
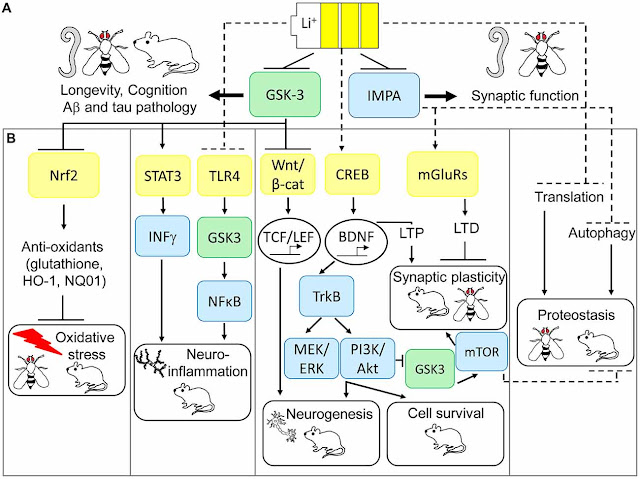
suggest in the book that lithium, clozapine, and possibly a few anticonvulsants
may be considered disease-modifying rather than symptomatically effective or
palliating medications. This recent paper presents his latest ideas on the
subject.
In his paper he
is much more specific. His premise is
that there are disease-modifying drugs and drugs that only treat symptoms and
that nearly all psychiatric drugs fall into the latter category. He reviews his
rationale for these classifications and emphasizes the lack of understanding of
pathophysiology of mental illnesses as a main reason for this deficiency. His
talking points are ideal newspaper headlines and will probably are easily
assimilated by many who don’t know much about psychiatry or medicine. This blog post is an elaboration of this story.
In order to
build those arguments, let me start with a brief introduction to
rheumatology. My personal introduction
to that field occurred in medical school when I had my first acute gout attack
and had a medicine attending who was a rheumatologist and two senior medicine
residents who aspired to and eventually became rheumatologists. I happened to
be at the medical school with one of the top experts in the field Daniel J.
McCarty. MD. Rheumatology in general
looks at inflammation in the narrowest sense in joints but more broadly in the
body and in multiple organ systems. Rheumatologists are experts in all forms of
arthritis but also systemic illnesses with joint manifestations like systemic
lupus erythematosus (SLE) and rheumatoid arthritis (RA). The American College of Rheumatology lists
the diagnostic criteria for 20 major groups of illnesses on their web site with additional criteria
for subclassification.
Why should
psychiatrists have an interest in rheumatology?
My initial interest was in the diseases themselves as well as the
classification system. Like psychiatric categorial diagnoses, the rheumatology
classification system is criterion based, based on expert consensus and ongoing
scientific review, and the sensitivity of the criteria are adjusted according
to what is clinically indicated. For example, a category could be adjusted to
be more inclusive with more false positives – if it was important to identify
early disease stages and prevent progression in the future. The disease categories are important to
psychiatrists because their overlap with psychiatric diagnoses. For example, neuropsychiatric SLE (NPSLE) is
defined as the usual symptoms of SLE with a central nervous system
manifestation like seizures, psychosis, or cognitive problems. It is in the differential diagnosis of
patients with psychosis. In addition, there are currently active hypotheses
about the role of inflammation in the pathophysiology of depression, psychosis,
and neurocognitive disorders at a level that is far below the threshold for
overt rheumatological disease.
The similar
classification system brings up similar concerns in rheumatology relative to
psychiatry. The issue of classification versus diagnosis for example. In a
recent review of that issue the problem described in rheumatology by a group of
experts is basically the same problem encountered in psychiatry:
“Rheumatologists
face unique challenges in discriminating between rheumatologic and non-rheumatologic
disorders with similar manifestations, and in discriminating among rheumatologic
disorders with shared features. The
majority of rheumatic diseases are multisystem
disorders with poorly understood etiology; they tend to be heterogeneous in their
presentation, course, and outcome, and do not have a single clinical,
laboratory,pathological,
or radiological feature that could serve as a “gold standard” in support of diagnosis
and/or classification.”
(3)
Psychiatry
or the equivalent term could be substituted for rheumatology, rheumatologic, or
rheumatic in the above paragraph without skipping a beat. Before the current
pandemic many rheumatology clinics were treating patients with symptoms that
could not be clearly attributed to rheumatic disease. In some cases, about 1/3 of patients were in
that category (4). The issue is
complicated by the fact that non-rheumatic origins of some of these symptoms
need to be recognized and addressed (5). The difficulties associated with rheumatic
diseases have led to “spectrum” descriptions of illness but as far I can tell
no push for dimensional rather than categorical diagnoses. There has also been
a concern about recognition in primary care settings with delayed referral to
rheumatologists (6).
Disease
complexity is difficult to address and rheumatologists like psychiatrists see a
number of conditions that do not remit, are progressive and can be fatal and/or
very disabling, and for which there are few good treatments. It is common in
psychiatry to see patients with rheumatoid arthritis who are treated on a
chronic basis with low dose prednisone – where the dose is adjusted according
to disease activity and degrees of complications from the medication. In other words,
the focus of treatment is symptomatic rather than curing or modifying the
course of the disease.
Unlike
psychiatry, rheumatology had an early focus on disease modification and using
the term “disease modifying” drugs. The
earliest reference to “disease-modifying” in PubMed that I could find was 1976
(7).
But
the connections to subsequent papers from that original paper seemed to stop in
the 1980s. That suggests to me that
there was an evolution of the terms and the medications used as DMARDS. Searching through modern medicine texts like
UpToDate shows that most of the references to disease-modifying medications is
focused on rheumatology diseases, multiple sclerosis and some other neurological
illnesses, and a few rare conditions. In
some cases, the focus is on a complication is a single organ system or an
intermediate phenotype of the main disease.
In
a paper specifically written about the term in rheumatology, Buer (8) describes
the concept of disease modifying anti-rheumatic drugs or DMARDS
beginning in the 1970s with the goal of preventing bone erosion from rheumatoid
arthritis. Use of the term increased over the next two decades outlasting
several competing terms. The early
purpose was to distinguish between medications that could slow or modify the
progression of disease and those that provided symptomatic relief.
Another
potential reason that the disease-modifying was developed in areas of medicine
where inflammation and immunological mechanisms where thought to play a part in
disease pathology was the longstanding and widespread use of glucocorticoids
(GC). GC drugs like prednisone have been
used for 60 years, are used by a substantial portion of the population and that
use is growing (15). The purported mechanisms
of action have been clarified over time and are currently characterized as
genomic and non-genomic (cytosolic GC receptor mediated and
specific/nonspecific effects). The
effect occurs at the level of cytokines, cell membranes, and immune cells. The
disease modifying effects of GC were first described in 1995 and are thought to
be limited to bone loss in the early stages of rheumatoid arthritis.
Considering
the characteristics of an ideal medicine that is curative or preventive and the
definitions of a disease modifying drug there is a lot of room for
interpretation. Endocrinopathies come to
mind – specifically deficiency states where replacement therapy of thyroxine,
corticosteroids, growth hormone, or gonadal hormones corrects the deficiency state
that is some cases is life-threatening.
Diabetes mellitus is another example.
Correcting insulin deficiency culminating in human insulins designed to
provide more even coverage of glucose levels has resulted in a significantly
altered life span for juvenile onset diabetes and for adults. There are also
examples in cardiology both from the standpoint of longevity and secondary
prevention of heart attacks, strokes, and renal failure. But most of the
literature on disease modifying medications is focused on rheumatology and
multiple sclerosis (US).
Using
MS as an example, I compiled a table of all current FDA approved MS treatments,
the year of approval, and what is known about the mechanism of action
(MOA). The MOA in each case is taken directly
from the FDA approved package insert. In
the case of natalizumab, there were several paragraphs describing the purported
mechanism of action so I included a link to the package insert. The important
observation from this table is that in the case of all 18 FDA approved
medications – the mechanism of action is unknown. That statement is made in
various ways. For example, there may be a suggested hypothetical MOA but it is
just that. In the case of MS disease-modifying drugs are based on an unproven
hypothesis, rather than a known mechanism of action or theory. I have not
constructed a table for rheumatology disease modifying drugs but I expect the
same results based on the quotation from reference 3 above. Disease-modifying
drugs do not appear to be specifically designed to address and underlying MOA –
but are empirically determined based on hypotheses like every other drug.
FDA
approved drugs for MS and Mechanism of Action
|
Drug
|
Type
|
MOA
|
|
Glatiramer (Copaxone)
Approved 1996
|
SC Injection
|
“The mechanism(s) by which glatiramer
acetate exerts its effects in patients with MS are not fully understood.
However, glatiramer acetate is thought to act by modifying immune processes
that are believed to be responsible for the pathogenesis of MS.”
|
|
Interferon beta 1a (Avonex)
Approved 1996
|
IM injection
|
“The mechanism of action by which
AVONEX exerts its effects in patients with multiple sclerosis is unknown.”
|
|
Interferon beta 1b (Betaseron)
Approved 1993
|
SC injection
|
“The mechanism of action of BETASERON
(interferon beta-1b) in patients with multiple sclerosis is unknown”
|
|
Peginterferon beta 1a (Plegridy)
Approved 2014
|
SC injection
|
“The mechanism by which PLEGRIDY
exerts its effects in patients with multiple sclerosis is unknown”
|
|
Dimethyl fumarate (Tecfidera)
Approved 2013
|
Oral tab
|
“The mechanism by which dimethyl
fumarate (DMF) exerts its therapeutic effect in multiple sclerosis is
unknown. DMF and the metabolite, monomethyl fumarate (MMF), have been shown
to activate the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in
vitro and in vivo in animals and humans. The Nrf2 pathway is involved in the
cellular response to oxidative stress. MMF has been identified as a nicotinic
acid receptor agonist in vitro.”
|
|
Fingolimod (Gilenya)
Approved 2010
|
Oral cap
|
“Fingolimod is metabolized by
sphingosine kinase to the active metabolite, fingolimod-phosphate.
Fingolimod-phosphate is a sphingosine 1-phosphate receptor modulator, and
binds with high affinity to sphingosine 1-phosphate receptors 1, 3, 4, and 5.
Fingolimod-phosphate blocks the capacity of lymphocytes to egress from lymph
nodes, reducing the number of lymphocytes in peripheral blood. The mechanism
by which fingolimod exerts therapeutic effects in multiple sclerosis is
unknown, but may involve reduction of lymphocyte migration into the central
nervous system.”
|
|
Teriflunomide (Aubagio)
Approved 2012
|
Oral tab
|
“Teriflunomide, an immunomodulatory
agent with anti-inflammatory properties, inhibits dihydroorotate dehydrogenase, a
mitochondrial enzyme involved in de novo pyrimidine synthesis. The exact
mechanism by which teriflunomide exerts its therapeutic effect in multiple
sclerosis is unknown but may involve a reduction in the number of activated
lymphocytes in CNS.”
|
|
Alemtuzumab (Lemtrada)
Approved 2001
|
IV infusion
|
“The precise mechanism by which
alemtuzumab exerts its therapeutic effects in multiple sclerosis is unknown
but is presumed to involve binding to CD52, a cell surface antigen present on
T and B lymphocytes, and on natural killer cells, monocytes, and macrophages.
Following cell surface binding to T and B lymphocytes, alemtuzumab results in
antibody-dependent cellular cytolysis and complement-mediated lysis.”
|
|
Mitoxantrone (Novantrone)
Approved 2000
|
IV Infusion
|
“Mitoxantrone, a DNA-reactive agent
that intercalates into deoxyribonucleic acid (DNA) through hydrogen bonding,
causes crosslinks and strand breaks. Mitoxantrone also interferes with
ribonucleic acid (RNA) and is a potent inhibitor of topoisomerase II, an
enzyme responsible for uncoiling and repairing damaged DNA. It has a
cytocidal effect Reference ID: 3105100 on both proliferating and
nonproliferating cultured human cells, suggesting lack of cell cycle phase
specificity. NOVANTRONEâ
has been shown in vitro to inhibit B cell, T cell, and macrophage
proliferation and impair antigen presentation, as well as the secretion of
interferon gamma, TNFα, and IL-2”
|
|
Natalizumab (Tysabri)
Approved 2004
|
IV infusion
|
“The specific mechanism(s) by which
TYSABRI exerts its effects in multiple sclerosis and Crohn’s disease have not
been fully defined” additional
|
|
Dalfampridine (Ampyra)
Approved 2010
|
Extended-release tab
|
“The mechanism by which dalfampridine
exerts its therapeutic effect has not been fully elucidated. Dalfampridine is
a broad spectrum potassium channel blocker. In animal studies, dalfampridine
has been shown to increase conduction of action potentials in demyelinated
axons through inhibition of potassium channels.”
|
|
Ofatumubab (Kesimpta)
Approved 2009
|
SC injection
|
“The precise mechanism by which
ofatumumab exerts its therapeutic effects in multiple sclerosis is unknown,
but is presumed to involve binding to CD20, a cell surface antigen present on
pre-B and mature B lymphocytes. Following cell surface binding to B
lymphocytes, ofatumumab results in antibody-dependent cellular cytolysis and
complement-mediated lysis.”
|
|
Cladribine (Mavenclad)
Approved 1993
|
Oral tab
|
“The mechanism by which cladribine exerts
its therapeutic effects in patients with multiple sclerosis has not been
fully elucidated but is thought to involve cytotoxic effects on B and T
lymphocytes through impairment of DNA synthesis, resulting in depletion of
lymphocytes.”
|
|
Siponimob (Mayzent)
Approved 2019
|
Oral tab
|
“Siponimod is a
sphingosine-1-phosphate (S1P) receptor modulator. Siponimod binds with high
affinity to S1P receptors 1 and 5. Siponimod blocks the capacity of
lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in
Reference ID: 4409346 12 peripheral blood. The mechanism by which siponimod
exerts therapeutic effects in multiple sclerosis is unknown, but may involve
reduction of lymphocyte migration into the central nervous system.”
|
|
Ocrelizumab (Ocrevus)
Approved 2017
|
IV infusion
|
“The precise mechanism by which
ocrelizumab exerts its therapeutic effects in multiple sclerosis is unknown,
but is presumed to involve binding to CD20, a cell surface antigen present on
pre-B and mature B lymphocytes. Following cell surface binding to B
lymphocytes, ocrelizumab results in antibody-dependent cellular cytolysis and
complement-mediated lysis.”
|
|
Ponesimod (Ponvory)
Approved 2021
|
Oral tab
|
“Ponesimod is a sphingosine
1-phosphate (S1P) receptor 1 modulator that binds with high affinity to S1P
receptor 1. Ponesimod blocks the capacity of lymphocytes to egress from lymph
nodes, reducing the number of lymphocytes in peripheral blood. The mechanism
by which ponesimod exerts therapeutic effects in multiple sclerosis is unknown,
but may involve reduction of lymphocyte migration into the central nervous
system.”
|
|
Diroximel fumarate (Vumerity)
Approved 2013
|
Oral delayed release capsule
|
“The mechanism by which diroximel
fumarate exerts its therapeutic effect in multiple sclerosis is unknown. MMF,
the active metabolite of diroximel fumarate, has been shown to activate the
nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in vitro and in
vivo in animals and humans. The Nrf2 pathway is involved in the cellular response
to oxidative stress. MMF has been identified as a nicotinic acid receptor
agonist in vitro.”
|
|
Ozanimod (Zeposia)
Approved 2020
|
Oral capsules
|
“Ozanimod is a sphingosine 1-phosphate
(S1P) receptor modulator that binds with high affinity to S1P receptors 1 and
5. Ozanimod blocks the capacity of lymphocytes to egress from lymph nodes,
reducing the number of lymphocytes in peripheral blood. The mechanism by
which ozanimod exerts therapeutic effects in multiple sclerosis is unknown
but may involve the reduction of lymphocyte migration into the central
nervous system.”
|
Effect
sizes for the above medications can be calculated from the package inserts. The typical active drug/placebo comparisons
include relapse frequency (per time interval), percentage of relapse-free
patients, reduction in relapse rates, time to first or second relapse,
progression free days, and numbers of new Gadolinium enhancing lesions on MRI
scan. This data is also plotted on survival curves. The calculations will be
made at some point and compared to similar data for lithium and selected DMARDs.
With
that backdrop consider the main points in Dr. Ghaemi’s paper – that do go
beyond the disease-modifying concept:
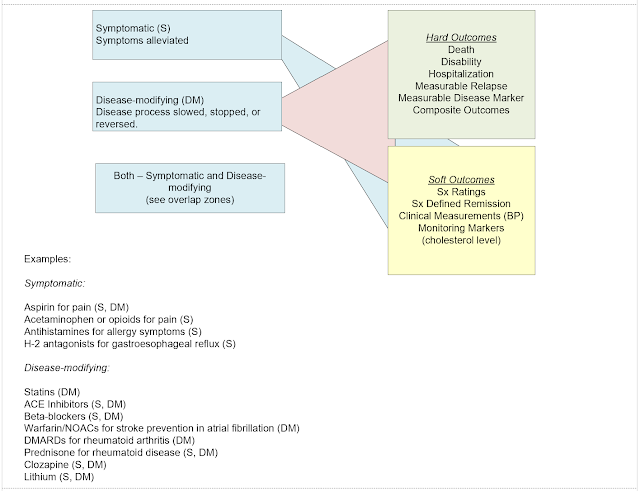
1. Symptomatic versus disease modification:
As I hoped to capture in the
preceding paragraphs – the issue of disease modification is a laudable goal but
a complex one. Even chemotherapy treatments that are curative vary in
effectiveness and can leave patients with complications from treatment that are
disabling or even fatal. There can also be at higher risk for future cancers
unrelated to the original treated cancer. Many symptomatic medications used on
a maintenance basis decrease mortality risk and disability (hard outcomes) even
though they are not disease-modifying. Anticonvulsant medications are a good
example. Where seizure risk in
generalized tonic-clonic seizures can be decreased it significantly reduces the
risk of sudden unexpected death in epilepsy (SUDEP) (9).
2.
Effect
size:
The
paper cites effect size as being problematic at two levels. The first is the actual calculated effect
size and the second in the end point – clinical metrics versus hard outcomes
measures. The first issue has been explored in the literature at an exhaustive
level. The unfortunate approach by many including a prominent epidemiologist
who suggested antidepressants had no effect and then later was a coauthor of a
paper showing an effect is a dichotomous one rather than an exploration of
reality. The issue is the same with all polygenic heterogenous diseases. There will be a group of responders, a group
of partial responders, and a group of non-responders. There is an associated overlay of placebo and
nocebo responders. And depending on the trial there are varying levels of severity,
heterogenous recruitment levels, and varying levels of support for research
subjects confounding the trials.
The
classification of effect sizes has also been problematic. Benchmarking of mild,
moderate, and robust effects sizes have been suggested but are generally
considered a weak approach. The actual
effects sizes can be calculated and discussed along with moderating factors. It
is possible to include different effect size calculations in the same table by specifying
the method used and the relevant parameters of the trial. Effect sizes that are considered low can become
significant over large populations.
3.
Disease
modification specific to psychiatry:
Lithium,
clozapine, and some anticonvulsants are known to be disease-modifying drugs in
psychiatry largely measured with the hard outcomes of time to relapse or number
of relapses in a set period of time. These medications address some purported
mechanisms at the hypothetical level since there is no widely accepted theory
about how they work and there are many hypothetical mechanisms. Considering the entire course of illnesses in
psychiatry medications that are not technically disease-modifying can make a
significant difference in hard outcomes. The best example that I can think of
and Luther Bell (10) described a mortality rate of 75% in a cohort of 40
patients admitted to McClean Hospital in 1849. Today with the advents of advances
in both medical treatment and electroconvulsive therapy the mortality in this
group of patients is essentially zero. Does preventing death qualify a
medication approach as disease-modifying?
If so, the modern medical treatment of catatonia (benzodiazepines,
antipsychotics, mood stabilizers) qualify. Another example is the use of long
acting injectable (LAI) antipsychotic medications. These medications clearly reduce the rate of
relapse in both schizophrenia and bipolar disorder. Does that qualify them for disease modifying
status even though the specific mechanism of action is unknown? Clinical psychiatry has clearly made progress
in terms of hard outcomes irrespective of where you draw the line on disease
modification.
4.
The
DSM is biologically invalid:
Somewhat
of a straw man – I don’t think there was ever a claim that it was. That said
there has been rumored validity markers of psychiatric disorders that have
apparently never been released by the DSM study groups and the most obvious
marker of robust medication effect has never been used. Further study of the RDoC and other proposed
alternate systems of classification do not seem any more biologically valid at
this point. At the minimum biological phenotyping may be useful and it
currently exists to a limited degree (catatonia). A lot of mileage has been made out of the
fact that a focus on the biological aspects of psychiatric illness has not yielded
any pertinent clinical information and that this somehow justifies increased psychosocial
research. That minimizes the issue of complex heterogenous diseases and what it
takes to understand them. Psychiatry compared with rheumatology is a good
example – but on the other hand psychiatric disorders are more intimately linked
to conscious states and those states can affect every level of interpretation
of a drug response.
5.
Clinical
trial design deficiencies
There
are many and I have already listed a few.
An additional deficiency is the general regulatory scheme that seems to
focus on getting a minimal efficacy signal.
Pharmaceutical companies are incentivized to complete these trials as
soon as possible. Anyone who has worked
as an investigator in a clinical trial knows that this is a frustrating process
largely due to the inclusion and exclusion criteria. There are pressures to
recruit the necessary patients as soon as possible. Randomization is a hurdle. What does it say when the number of people
declining participation in the study greatly outnumbers the people who have
been recruited? Many of them decline because
of randomization to possible placebo or an inability to be notified after the
study about whether they received placebo or not. At the design level, the
recruiting problem also can affect choices of comparator drugs and the doses of
those drugs. More long-term studies require more funding and retaining patients
in the study becomes an important task for researchers. Intent-to-treat analysis based on considering all
of the patients entering the protocol as the denominator in the study is
another limitation in that it does not resemble clinical practice where getting
to responder status as soon as possible independent of any particular drug is a
priority.
The
discontinuation design in maintenance studies of antidepressants were described
as a problem in terms of falsifiability.
Most of them show an active drug effect and apparently psychiatric
medications are the only class of drugs that the FDA allows to use this discontinuation
paradigm. The practical issue in terms of clinical treatment is what happens
when antidepressants are stopped. Some
early work in the pattern analysis of antidepressant response suggested that
the placebo effect faded over time but the active drug effect did not. Psychiatrists need to know what the treatment
scenarios are with drug discontinuation.
There
has not be enough discussion of registry and observational studies. The advantages
are that they use large data bases and can look at hard outcomes like relapse, hospitalization,
suicide, and other types of mortality.
It fits the current FDA regulatory category of Real World Evidence (RWE)
and Real World Data (RWD). The main
advantage is the population studied in the registry is not screened by inclusion
and exclusion criteria or by a participatory agreement and therefore more
accurately approximates a true clinical population (14). The time interval for RCTs is typically
limited by funding for a duration of years. Registry studies based on a
database can be much longer in duration and the data is a standard
administrative feature. Safeguards have been developed to reduce bias in
registry studies and some groups consider them to be a good indication of how a
medication works in real clinical settings. Although I have not seen it done,
registry studies could potentially confirm some of the effect sizes when
applied to much larger populations.
6 Academics
versus Industry versus Clinical Practice:
Closer collaboration between
the pharmaceutical industry and may be useful, but there will always be
significant conflict of interest issues.
The pharmaceutical industry is clearly looking for an efficacy signal
they can use to get FDA approval and market a drug. The trade-off is that these
are typically small studies with stringent inclusion criteria that can result
in later drug withdrawal due to complications noted only with greater exposure
in post-marketing surveillance. It is not clear to how this system will ever
produce medications that are disease-modifying versus those that are used to
treat symptoms.
An even larger problem is
that clinicians are typically an afterthought by the academics and
pharmaceutical industry. The job of
every psychiatrist is to see people who are acutely symptomatic and diagnose
and treat those people. Psychiatrists are currently under more constraints than
they have ever been. Managed care
companies demand that people are discharged from hospitals barely treated while
psychiatrists are concerned about adequate treatment of the symptoms that led
to hospitalization. There are very few –
if any clinical trials that apply to this scenario. In 22 years of acute inpatient care – my
estimate would be that about 5% of the people I treated would not be excluded
from a standard clinical trial. That
experience was reinforced by my experience as an investigator in clinical
trials of antidepressants, anxiolytics, antipsychotics, and Alzheimer’s
disease. From a clinician’s perspective, the main failure of drug development
is continuing to ignore real-world patients for an idealized clinical trials
process.
Concluding this post – I
hope that I have communicated alternate viewpoints that capture the broader clinical
landscape. It is not intended as a refutation of Dr. Ghaemi’s viewpoint and I don't consider anything in his paper to be controversial. What I am suggesting is that psychiatrists
need to know all of the viewpoints on these topics and why they exist in order succeed
in clinical settings. For example, they need to know how to use both
symptom-modifying and disease-modifying medications and the limitations of that distinction. They need to know the
limitations of any medication prescribed and how to rapidly determine when a
medication needs to be discontinued and a new medication or mode of therapy
initiated. They need to know about placebo and nocebo effects as well as the
entire range of side effects, effects on comorbid medical illnesses, and drug
interactions. And they need to know the relative merits of randomized clinical trials using intent-to-treat analysis and real-world observational and registry
studies. All of those knowledge is necessary
to treat complex polygenic illnesses that probably have many underlying
biological processes and that consideration is not limited to psychiatry.
That is the true state-of-the
art in the field. There is no royal road
to the truth and the current road is never easy. Many people go into psychiatry for that
reason, they get to know this body of knowledge and the associated decision-making
and they are very good at helping people with significant problems.
George Dawson, MD, DFAPA
References:
1: Ghaemi SN.
Clinical Psychopharmacology: Principles and Practice. New York, Oxford University Press 2019.
2: Ghaemi SN. Symptomatic versus
disease-modifying effects of psychiatric drugs. Acta Psychiatr Scand. 2022 Jun
2. doi: 10.1111/acps.13459. Epub ahead of print. PMID: 35653111.
3: Aggarwal R, Ringold S, Khanna D, Neogi T,
Johnson SR, Miller A, Brunner HI, Ogawa R, Felson D, Ogdie A, Aletaha D,
Feldman BM. Distinctions between diagnostic and classification criteria?
Arthritis Care Res (Hoboken). 2015 Jul;67(7):891-7. doi: 10.1002/acr.22583.
PMID: 25776731; PMCID: PMC4482786.
4: N. L. Maiden, N. P. Hurst, A. Lochhead, A. J.
Carson, M. Sharpe, Medically unexplained symptoms in patients referred to a
specialist rheumatology service: prevalence and associations, Rheumatology,
Volume 42, Issue 1, January 2003, Pages 108–112, https://doi.org/10.1093/rheumatology/keg043
5: Smythe HA. Explaining medically unexplained
symptoms: widespread pain. The Journal of Rheumatology. 2009 Apr
1;36(4):679-83.
6: Gran JT, Nordvåg BY. Referrals from general
practice to an outpatient rheumatology clinic: disease spectrum and analysis of
referral letters. Clinical rheumatology. 2000 Nov;19(6):450-4.
7: Gumpel JM. Cyclophosphamide, gold and
penicillamine--disease-modifying drugs in rheumatoid arthritis--tailored dosage
and ultimate success. Rheumatol Rehabil. 1976 Aug;15(3):217-20. doi:
10.1093/rheumatology/15.3.217. PMID: 968355
8: Buer JK. A history of the term
"DMARD". Inflammopharmacology. 2015 Aug;23(4):163-71. doi:
10.1007/s10787-015-0232-5. Epub 2015 May 23. PMID: 26002695; PMCID: PMC4508364
9: Pensel MC, Nass RD, Taubøll E, Aurlien D,
Surges R. Prevention of sudden unexpected death in epilepsy: current status and
future perspectives. Expert Rev Neurother. 2020 May;20(5):497-508. doi: 10.1080/14737175.2020.1754195.
Epub 2020 Apr 26. PMID: 32270723.
10: Leucht S, Helfer B, Gartlehner G, Davis JM.
How effective are common medications: a perspective based on meta-analyses of
major drugs. BMC Med. 2015 Oct 2;13:253. doi: 10.1186/s12916-015-0494-1. PMID:
26431961; PMCID: PMC4592565.
11: Bell, L. 1849. On a form of disease
resembling some advanced stage of mania and fever. Am. J. Insanity 6, 97–127.
12: Fava M.
Rational use of antidepressants. Psychother Psychosom 2014;83:197–204.
doi: 10.1159/000362803
13: Cosci F, Fava GA. Prescribing Pharmacotherapy
for Major Depressive Disorder: How Does a Clinician Decide?. Biomedicine hub.
2021;6(3):118-21.
14: Taipale H, Tiihonen J. Registry-based
studies: What they can tell us, and what they cannot. Eur Neuropsychopharmacol.
2021 Apr;45:35-37. doi: 10.1016/j.euroneuro.2021.03.005. Epub 2021 Mar 25.
PMID: 33774390.
15: Frew AJ. Glucocorticoids. In: Clinical immunology: principles and practice,
5th edition. Rich RR, Shearer WT, Schroeder HW, Frew AJ, Weyland CM,
editors. Amsterdam: Elsevier; 2019. p 1165-1175
Supplementary: This post is another work in progress. I hope to calculate effects sizes of the above medications for MS, another table for rheumatic conditions (RA or SLE) and compare them to effect sizes for lithium, clozapine, valproate, and carbamazepine. I am interested in the longest RCTS and registry studies that examine these problems. If you have favorite studies please post the references here or email them to me.
Image credit: My wife took this photo of the Bong Bridge between Duluth, MN and Superior, WI. It is an expansive structure and hope I communicated that concept in the above writing.