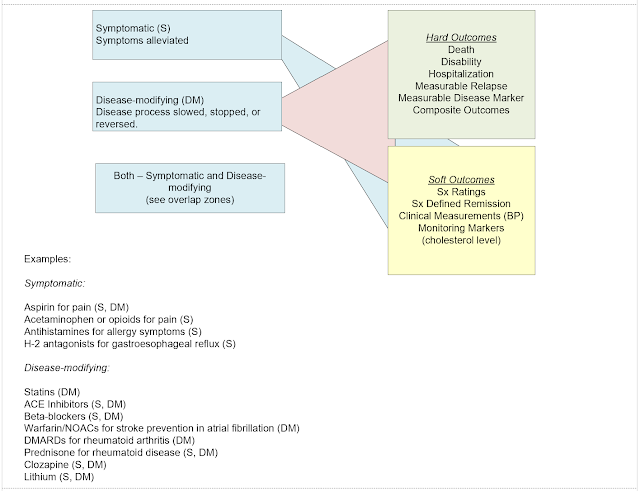
My last post was not satisfactory after I read thorough it
several times. I decided to diagram the
concepts of symptomatic and disease modifying with the suggested parameters and
cite a few specific examples in the diagram.
An obvious problem with the definitions as used by Professor Ghaemi is
that it involved a lot of inductive reasoning on the pathophysiology side of
the equation.
How can you say a medication is affecting underlying
pathophysiology if it is unknown? Much
pathophysiology of complex disease is at the hypothetical rather than
theoretical level. The hypotheses are restated over time, but there are no widely
accepted theories. That has resulted in measurable proxies for pathophysiology
being used like serum biomarkers for rheumatological disease and brain plaques
for active disease and remissions in multiple sclerosis.
The problem with that approach is highlighted with the
recent controversy in the FDA approval of aducanumab ( an anti-beta~amyloid antibody) for Alzheimer’s Disease or mild
cognitive impairment. The biomarker of
PET visualized beta~amyloid plaques were significantly improved in two clinical
trials relative to placebo. Both of
these trials were terminated early when it became apparent, they would not
reach primary efficacy endpoints. In this case, the drug did not reach clinical
efficacy as a disease-modifying drug and it is effective against a biomarker
that may not be a true marker of the pathophysiology of Alzheimer’s
Disease. Additionally, the drug was
associated with amyloid-related imaging abnormalities (ARIA) at a significantly
higher rate than placebo. These lesions
were thought to represent vasogenic edema and microhemorrhages and necessitate
careful screening at baseline for preexisting vascular disease.
There are biases in the literature on where
the term was used. Disease-modifying
seems to be associated primarily with rheumatological and some specific
illnesses like multiple sclerosis. In
other areas it is mentioned primarily as an absence as in: “there are no
disease-modifying drugs for this condition”.
What are the main points that I tried to incorporate into the diagram?
1. Neither the disease-modifying or symptomatic interventions are 100% effective. With any complex, polygenic illness there will be non-responders, partial responders, and optimal responders. In many studies the optimal responders will meet study criteria for remission – but that also happens in clinical practice. For example, it is common to assess people undergoing antidepressant treatment and find that from a subjective standpoint their depressive symptoms are gone and they feel like they are back at their baseline. Of course, in randomized clinical trials there is an intent-to-treat analysis that counts study drops outs in the denominator no matter what the cause. In clinical practice that group would be provided with an alternate treatment.
2. There can be overlap between disease-modifying and symptomatic treatment. There are a few examples in the graphic, but prednisone for rheumatoid arthritis is probably the best example. It treats acute joint and systemic inflammation while preventing bone erosion early in the course of illness. There are many drugs that are either symptomatic or disease-modifying.
3. Many of the
outcomes are not cleanly separable.
Symptom ratings and symptom defined remission can be associated with
hospitalizations, disease markers, and composite outcomes. Measurable disease
markers or biomarkers are certainly preferable, but as noted in the case of
aducanumab there may be unexpected findings suggesting other important
underlying pathophysiology. In the case
of complex diseases we are learning more and more that many genes and many gene
networks are potentially involved. In
that case – a final common pathway or a parsimonious analysis should not be
expected. We appear to be at the very
early stages of being able to analyze these complex interactions.
No matter how you parse it – the definitions considered
above do not allow for a very clearly defined category of disease-modifying and
symptomatic. Does regulatory language
from the US Food and Drug Administration (FDA) or the European Medicines Agency
(EMA) help? The standard for disease-modifying
approvals apparently changed before 2019 (2).
Up until that time the standards were largely descriptive like delaying
disability or slowing the progress of illness.
The authors note an uneven approach to the term disease-modifying with
a tendency to use it in rheumatic diseases but less so in neurodegenerative
diseases. They attribute that to disease-modifying being an inferential
concept because “changes in the brain cannot be directly observed”.
This is an important concept because it applies to all of
the hypothetical mechanisms of action of most drugs that exist. In the previous post I pointed out that all
of the disease modifying drugs for multiple sclerosis have no widely accepted
theories about their mechanism of action.
The hypotheses may be listed but more commonly are listed as unknown or
not fully understood. The same is true
for the disease-modifying drugs in psychiatry listed by Ghaemi in his recent
paper (4). Various signaling systems are
cited, but the reality is there is no widely accepted mechanism of action and
more importantly no mechanistic way to explain lithium nonresponse.
The best way to approach disease-modifying drugs in general
and more specifically in psychiatry is to discuss the hypothetical mechanisms
of action and the implications of those mechanisms. That is the focus of research and the basis
for many purported biomarkers of disease in psychiatry but clearly in many
other fields of medicine. With the advent of genomics we are also witnessing a
necessary paradigm shift away from simple explanations.
George Dawson, MD, DFAPA
References:
1: Rabinovici GD.
Controversy and Progress in Alzheimer's Disease - FDA Approval of Aducanumab. N
Engl J Med. 2021 Aug 26;385(9):771-774. doi: 10.1056/NEJMp2111320. Epub 2021
Jul 28. PMID: 34320284.
2: Morant AV,
Jagalski V, Vestergaard HT. Labeling of Disease-Modifying Therapies for
Neurodegenerative Disorders. Front Med (Lausanne). 2019 Oct 17;6:223. doi:
10.3389/fmed.2019.00223. PMID: 31681780; PMCID: PMC6811601.
3: Schubert, K.O.,
Thalamuthu, A., Amare, A.T. et al. Combining schizophrenia and
depression polygenic risk scores improves the genetic prediction of lithium
response in bipolar disorder patients. Transl Psychiatry 11, 606
(2021). https://doi.org/10.1038/s41398-021-01702-2
4: Ghaemi SN.
Symptomatic versus disease-modifying effects of psychiatric drugs. Acta
Psychiatr Scand. 2022 Jun 2. doi: 10.1111/acps.13459. Epub ahead of print.
PMID: 35653111
5: Alda M. Lithium
in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol
Psychiatry. 2015 Jun;20(6):661-70. doi: 10.1038/mp.2015.4. Epub 2015 Feb 17.
PMID: 25687772; PMCID: PMC5125816.
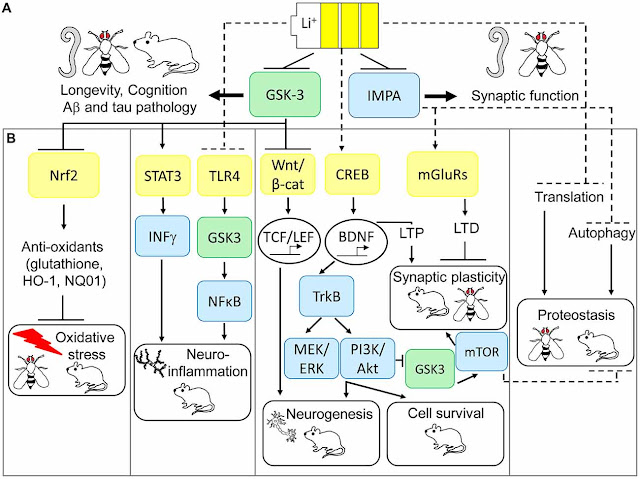
6: Kerr F, Bjedov I
and Sofola-Adesakin O (2018) Molecular Mechanisms of Lithium Action: Switching
the Light on Multiple Targets for Dementia Using Animal Models. Front. Mol.
Neurosci. 11:297. doi: 10.3389/fnmol.2018.00297
Graphic Credit:
Reference 6 and the following copyright and Open Access license: