For starters GLP-1 agonists are drugs like Ozempic and Weygovy. See this post for a current list. It is hard not to hear about them since they are heavily hyped in just about every form of media. They are being touted as a cure for just about everything. Various celebrities are either promoting them or denying that a dramatic weight loss was associated with their use. Some in the weight loss and exercise industry are pushing back with statements about side effects and rapid weight gain if you ever stop taking them. The sales of these drugs is a windfall for the pharmaceutical industry and current pricing means that other businesses that make money from rationing access to medical care and medications will be trying to prevent their use. I thought I would post a contrast today between the latest review of conditions these medications have been researched for and a new paper that suggests they may increase the frequency of psychiatric disorders.
The rest of the title comes from my experience in many
medical settings over the decades. Any
time a medication is commonly prescribed you can count on someone saying “We
should just put it in the drinking water.”
Examples over the years have been amoxicillin, H-2 blockers like
ranitidine, statins, beta blockers, lorazepam, and even haloperidol. It all depends on the prescription frequency
in a particular setting. At the rate GLP-1 agonists have been hyped - somebody
is saying it somewhere. The irony in
that statement is that many medications are now in the water supply and not
doing anything for anybody.
When I describe this group of drugs as hyped that is exactly
what I mean. The only comparable hype has been for cannabis and psychedelics/hallucinogens. Typical newspaper headlines about GLP-1s say
they are wonder drugs and go on to describe them as indicated for several
conditions ranging from addiction to Alzheimer’s disease. Currently 5.4% of all medication
prescriptions in the United States are for GLP-1 agonists. These drugs have been
around for 20 years and during that time transitioned from use primarily
for Diabetes Mellitus Type 2 to weight loss. Despite all the clinical trials
and experience with them I do not think the final verdict is in and the main
papers relevant to this post will illustrate why.
The first paper (1) is a large observational study using
databases from the Veterans Administration (VA) health care system (1). The authors describe the rationale of their
study as looking at the real-world outcomes of the use of GLP-1 agonists – both
the positive effects and adverse outcomes. They had an N of 1,955,135 followed
for a median of 3.68 years looking at 175 health outcomes. The authors use an interesting
methodology. Patients were recruited
based on incident use of a medications for Type 2 diabetes mellitus (T2DM) between
October 1, 2017 and December 31, 2023.
That created 4 groups based on the medical treatment of T2DM) including GLP-1 agonists
(N= 232,210), sulfonylureas (N= 247,146), Dipeptidyl peptidase-4 (DPP-4i)
inhibitors (N= 225,116), and SGLT2i inhibitors (sodium-glucose cotransporter 2
inhibitors) (N= 429,172). There was also
a treatment as usual (TAU) group (N= 1,513,896) with Type 2 DM who took
non-GLP-1 antihyperglycemics between the study dates of October 1, 2017 and
December 31, 2023. As a point of reference,
I have included a table of the medications in each class used for T2DM.
|
Glucagon-like
peptide 1 (GLP-1) agonists |
exenatide
(Bydureon) exenatide
(Byetta) liraglutide
(Victoza) liraglutide
(Saxenda) dulaglutide
(Trulicity) semaglutide
(Wegovey) semaglutide
(Ozempic) semaglutide
(Rybelsus) tirzepatide
(Mounjaro) tirzepatide
(Zepbound) |
|
|
|
|
Sulfonylureas |
Glipizide Glimepiride Glyburide |
|
|
|
|
dipeptidyl peptidase
4 (DPP4) inhibitors |
alogliptin (Nesina,
Vipidia) sitagliptin
(Januvia) saxagliptin
(Onglyza) linagliptin
(Tradjenta) |
|
|
|
|
sodium−glucose
cotransporter-2 (SGLT2) inhibitors) |
bexagliflozin
(Brenzavvy) canagliflozin
(Invokana) dapagliflozin
(Farxiga) empagliflozin
(Jardiance) ertugliflozin
(Steglatro) |
This study was designed to assess groups on 175 health
outcomes from these treatment cohorts compared with two control groups. One control group was a composite of equal
numbers of diabetic subjects using oral hypoglycemics and the other control
groups was diabetics who continued GLP-1 agonists that they had already been
started on. Results varied but generally
the health outcomes measured were significantly improved on the GLP-1 agonists
compared with the controls and across categories. For example, when GLP-1 agonists were compared
with the sulfonylurea, DPP4, and SGLT2 classes outcomes were improved in
13.14%, 17.14%, and 11.43% of the outcomes respectively.
Risk of adverse outcomes were 8%, 7.43%, and 16.57% in the
same order. Those adverse events in
aggregate included: nausea and vomiting, gastroesophageal reflux disease
(GERD), sleep disturbances, bone pain, abdominal pain, hypotension, headaches,
nephrolithiasis, and anemia.
When comparing the addition of GLP-1s to treatment as usual
(the composite control) better outcomes were observed in 24% and increased risk
of adverse outcomes in 10.86% of outcomes.
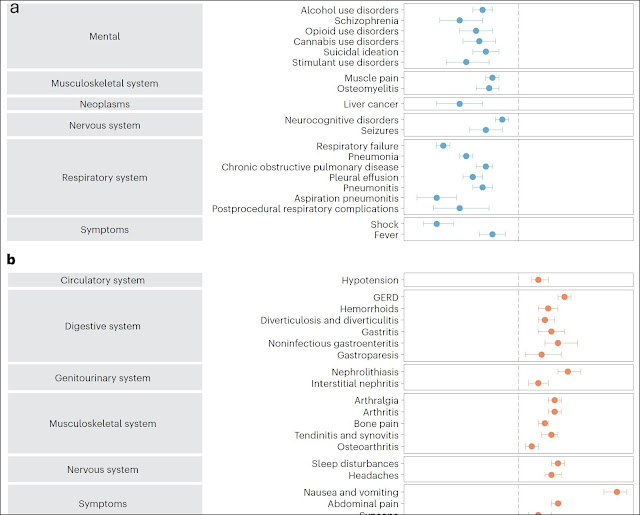
The reduced risk of several CNS disorders were estimated by
hazard ratios and they were modestly decreased for alcohol use disorder,
cannabis use disorder, stimulant use disorder, opioid use disorder, suicidal
ideation of self-injury, bulimia, schizophrenia, seizures and neurocognitive
disorders. Risk reductions were in the
10-16% range.
The authors of this paper use several graphing techniques to present their data. They graphed hazard ratios for both improved and adverse outcomes and made negative log transformed Manhattan plots as a measure of statistical robustness as alternate graphing technique. The paper is open access and I encourage reading the paper to see these data presentations. I included a partial Forest plot at the top of this post to illustrate some of these graphs and the outcomes they measured. The blue dots indicate reduced risk relative to controls and the orange dots indicate increased risk (calculated as hazard ratios (3).
The strength of this study is that it summarizes a large
amount of data across a VA database.
Since it is administrative data it is collected in nonstandard way and
the diagnoses are not necessarily made by experts - this data may not be as robust as a prospective randomized clinical trial. The population was older white veterans and
that may be a factor when considering pleotropic effects. The authors conclude that the GLP-1 agonists
had broad pleotropic effects based on the spectrum of positive results
and preclinical work. They emphasize the
positive results for neuropsychiatric diseases and disorders. They discuss the issue of suicidal behavior
and point out that earlier studies raised concerns to the point that the
European Medicines Agency investigated and found no evidence for causality. This study showed decreased suicidality and
possible antidepressant effects. The
results generally showed significant positive effects on outcomes across major
disease categories with a clear group of adverse effects.
For comparison there is a recent large retrospective cohort study (2) that uses deidentified data on patients from 66 different health care organizations. This appears to be a database with a commercial purpose, but I cannot identify what that purpose might be based on their web site. In their rollover map, most of the deidentified patients in this database are Americans. The study was approved by an IRB in China and I assume that is where the analysis takes place. The study was focused on examining the effects of GLP-1 agonists on patients being treated for obesity. Subjects were selected for a diagnosis of obesity and incident use of a GLP-1 agonist. It was a retrospective cohort analysis similar to the first study but propensity score matching was done to pair treatment subjects more closely with controls. Exclusion criteria included use of any other weight loss drug and any psychiatric diagnosis or significant symptom like suicidality.
The main results of this study are summarized in 3 tables in
the body of the paper (Tables 2, 3, and 4).
Psychiatric outcomes were measured over a period of 5 years and the percentage
of patients with major depression, any anxiety, any psychiatric disorder and
suicidality (ideation or behaviors) we measured at 6 months, 1 year, 3 years,
and 5 years. The cumulative incidences
of disorders and suicidality increased over these intervals. Hazard ratios were calculated compared with
the control population and they were generally doubled.
Results stratified on demographic factors and GLP-1 agonist
potency showed that both sexes had higher than expected psychiatric morbidity
associated with GLP-1 agonist use but that women had significantly higher hazard
ratios across all categories. Age was inversely correlated with older
populations having lower risk of psychiatric comorbidity. Finally, the potency
of the GLP-1 agonist directly correlated with potency of the GLP-1 agonist and
time of exposure. The authors discuss
the limitations of their study and implications for future use and study.
Both studies generally illustrate some of the advantages and problems of conducting
large clinical trials. The numbers in the hundreds of thousands or million plus
range would be very difficult if not impossible to conduct randomized clinical
trials on. It is manageable using the naturalistic
retrospective designs employed here commonly referred to as real world designs. The obvious limiting factor is expense and
the methodological problem of drop outs over time. In these specific cases the first study is selecting
a subject cohort based on a diagnosis of diabetes mellitus type 2 (DMT2) and
the second obesity. Both are
heterogeneous populations with some overlap.
If I was influenced at all by some of the current psychiatric literature,
I might suggest transdiagnostic features common to both but the
importance of that term seems inflated relative to common medical diagnostic
formulations. Instead - I will use the
parlance of medical trials and point out that there are signals in both papers. Those signals are both good and not so
good. In the first paper there were
clearly improvements in many medical outcomes when T2DM was treated with GLP-1
agonists in about 25% of the conditions studied and adverse outcomes in about
10%. Improvement occurred in conditions
outside of the endocrine/metabolic sphere including some psychiatric conditions.
In the second study, significant increases in psychiatric conditions were noted
to occur associated with GLP-1 agonist potency and total exposure in a
population selected for obesity treatment.
The authors are careful to point out that obesity and metabolic syndrome
may be a risk factor for mood disorders and they provide an excellent
discussion of how trial design and patient selection may have affected these
results.
When these trials are reported in the news, they are
generally not reported as showing modest results. Side effects are typically ignored. I have not heard anything about the study
that showed that increased rather than decreased psychiatric morbidity may be a
possible outcome. The media generally
reports them as miracle drugs and patients with the best possible
results are given as examples.
GLP-1 agonists are clearly serious medications with
potentially serious adverse effects. The
prescription of these medications requires close monitoring and thorough
patient education. If I was prescribing these medications today - in the informed consent discussion I would include the potential for modest outcomes, potentially increased psychiatric side effects, the general potential for side effects, and why outcomes may be variable. I would also make sure to let people know that long term outcomes at this point are not known with any degree of certainty.
George Dawson, MD, DFAPA
References:
1: Xie Y, Choi T,
Al-Aly Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat
Med. 2025 Jan 20. doi: 10.1038/s41591-024-03412-w. Epub ahead of print. PMID:
39833406.
2: Kornelius E, Huang
JY, Lo SC, Huang CN, Yang YS. The risk of depression, anxiety, and suicidal
behavior in patients with obesity on glucagon like peptide-1 receptor agonist
therapy. Sci Rep. 2024 Oct 18;14(1):24433. doi: 10.1038/s41598-024-75965-2.
PMID: 39424950; PMCID: PMC11489776
3: Spruance SL, Reid
JE, Grace M, Samore M. Hazard ratio in clinical trials. Antimicrob Agents
Chemother. 2004 Aug;48(8):2787-92. doi: 10.1128/AAC.48.8.2787-2792.2004. PMID:
15273082; PMCID: PMC478551.