Monday, January 15, 2018
A Short (11 minute) Film About Alcoholism - Breakfast Wine
"In Ireland, they say it takes just 3 alcoholics to keep a small bar running in a country town....."
I ran into this film last week and was impressed enough to write this post about it. It is an award winning short film about four people in a pub in rural Ireland and events that transpired on a certain day. Given the availability and brevity of the film, I encourage anyone interested to view the video before reading this post.
You can go to any number of Saturday Night Live skits and see alcoholics ridiculed. This film takes the problem more seriously. What you see will depend on your experience observing and interacting with people who have an alcohol problem.
To set the scene, the film begins with the quote posted at the top of this page. We see two middle-aged men standing outside a pub waiting for it to open. The owner shows up. They all enter and the two men who were waiting commence drinking pints of Guinness. Over the duration of the time lapse in the film they each drink 6 pints of Guinness - the first two in what seems fairly rapid succession.
After they put down about two pints a young woman enters, asks them about the availability of wine. After getting their recommendations she proceeds to drink the first glass rapidly. Over the time lapsed in the film she drinks a total of 4-187.5 ml bottles. The pub owner and the two men in the bar seem impressed with her ability to drink, but they are also impressed with her ability as a ranconteur. She tells a fable about getting rid of all motor transport and lining people up against the wall and shooting them. She moves on to describe severe physical abuse by her husband and shows lacerations on her wrists where she was tied up on the garage floor. She tells them what her husband was saying to her when he became abusive and does not miss a beat. She sarcastically dismisses her rant by saying "I should have seen it coming." At that point she says good bye and the pub owner tells her what the opening time for the pub is every day. She thanks him for the information and walks out. The men are clearly impressed with her and one of them longingly touches the stool where she was sitting.
At the level of entertainment, I can see why this is an award winning short. The writing and acting are good. The woman in the scene, actress Ruth Bradley is a compelling screen presence. She plays this role perfectly. It is a plausible scene from any bar - Irish or American. From the standpoint of an addiction psychiatrist - what is wrong with this picture?
The alcohol consumed per unit time is a red flag. The CDC defines binge drinking as probably occurring if a woman consumes 4 or more drinks or a man consumes 5 or more in 2 hours. The time span of this film may be subject to debate but I counted 6 pints of Guinness per man or 9.6 standard drinks and 750 ml wine (4 - 187.5 ml bottles) or 5 standard drinks for the woman. It is clear from the depiction by the actors that they are drinking these beverages at times like water. In bars or pubs bad things tend to happen when the patrons are binge drinking. Binge drinking alone whether it is associated with a diagnosis of alcohol use problems has associated mortality and morbidity per the CDC site.
There is a high tolerance for unusual behavior in the pub. One of the men leaves and his behavior is discussed among the remaining people. In another scene marking the sixth pint he becomes irate with his associate and that behavior is translated as an acknowledgement that he does want another pint. The woman had two discrete rants about motor vehicles and the violence she has sustained by her husband and immediately resumes a normal even joking manner. The men are intensely interested in this woman, she breaks up their routine, is attractive to them and is charismatic. Their response to her description of the violence she has sustained and even the presentation of her wrist lacerations is definitely muted. No one is interested in the violence or her newly acquired wounds, everyone is interested in moving on as soon as possible.
The dynamic that jumps out at me whether I am listening to heavy drinkers talk about relationships or observing them first hand in bars is grandiosity. Grandiosity can be a feature of mood disorders or narcissism. One theory of grandiosity in narcissism is that there is an inadequate mental representation of affirming objects representing attributes or real relationships in the environment. If people with those attributes are absent or the people present have an inadequate positive affiliation with the person in question they can form their own representations. That leads to grandiosity and narcissism as an amplification of a deficient process with more realistic balances. In alcoholism this can occur as a reaction to the hopelessness of the addiction and its sequelae. As an example, a bar full of middle-aged men with alcoholic liver disease betting on who is going to die first. Sometimes it occurs on a larger scale - the family that supports their father's grandiose statements about drinking himself to death during a hospitalization for recurrent hepatic encephalopathy from alcoholic cirrhosis. The person involved comes across as though they are indestructible - but at a deeper level they cannot reconcile the severity of their illness and their inability to stop drinking.
That is what I think the female character brings to this scene. She is clearly in an abusive marriage that she fled earlier the same day that she comes into the pub. She does not appear to be traumatized at all until she demonstrates the injuries and even then she is loudly mocking her husband and eventually herself: "I should have seen it coming!" The men seem transfixed on this story - unable to challenge it or accept the reality of her status as an abused wife.
I saw one comment posted on the YouTube site from a person who watched this film on an airline flight. He was left thinking about the lives of the people in the film and what happened to them. That is natural enough and something I wondered when I encountered a heavily intoxicated young woman in Boston and tried to help her. To me it is always a lesson in the dynamics of heavy alcohol use. There is an element of intoxication involved, but there is more than that driving a lot of the interpersonal behaviors and what you see happening in a pub or bar.
Defensive behavior about the inability to stop can be part of that.
George Dawson, MD, DFAPA
Sunday, January 14, 2018
Lithium for Depression.....
I bought a copy of Manic Depressive Illness when it first came out in 1990. One of the more interesting aspects of the book was the commentary on the use of lithium monotherapy for unipolar depression. That discussion is limited to about three pages and reviewed the work to date. In the opening paragraph there is this astonishing line: "Overall, the result of open studies suggest that lithium is as effective in preventing unipolar illness as it is in preventing bipolar illness." At the time there were 4 controlled studies (3-6) looking at the issue of maintenance therapy in mixed unipolar and bipolar groups. Three of the four studies showed no difference between groups. The fourth study was inconlusive due to a high dropout rate. Subsequent analyses by Schou and Baldessarini and Tohen concluded that the protective effects of lithium in preventing recurrent depressive episodes was good. In Schou's reanalysis he showed that the relapse rate in one year on lithium for unipolar depression was 22% (compared with a 20% relapse rate for bipolar disorder) and the rates for antidepressants at one year were 35% for unipolar depression and 65% for bipolar disorder (relative to placebo of 67-68% relapse rate).
Since that early open research there has been only randomized controlled clinical trial (RCT) of lithium versus placebo antidepressant augmentation (7). In that study 7/15 patients treated with placebo relapsed and one suicided. In the lithium treated group 0/14 relapsed. The authors recommended that patients who respond to lithium augmentation be maintained on it for at least 6 months. Additional clinical parameters of interest in the treated group was an average lithium dose of 980 mg, an average Li level of 0.65 mmol/L, and a response time of 17.5 days in acute treatment. This is interesting because many psychiatrists using lithium augmentation were taught to stop at 600 mg/day and accept the associated lower levels.
My point in the introductory paragraph here is that it has been known for some time that lithium may have a role in maintenance of unipolar depression in addition to bipolar depression - even though it is hardly ever used that way int he United States. In the US, patients typically endure a long series of antidepressant trials or augmentation strategies if the initial trails are ineffective. There is always the problems of whether the antidepressant has lost its effect or not and the associated difficulty of trying to determine what other factors may be operative. Lithium has been used for the past three decades as an augmenting agent - added to antidepressants. Typically a lower dose is used (600 mg/day) and that may offer a lower chance of toxicity and less need for monitoring blood levels.
Against this backdrop, a very interesting paper by Tiihonen, et al came out last year (2). For reasons that will follow, I consider this to be one of the most important papers for clinical psychiatrists from 2017. The authors provide a sound rationale for their study, specifically the advantages of a large scale epidemiological/observational study over an RCT or the Cochrane meta-analysis of RCTs. That meta-analysis (like most Cochrane meta-analyses) concluded that the number of subjects included was too small to come to a statistically significant conclusion.
The study was conducted in Finland. The authors point out that each citizen has a unique identifier that facilitates the design of large inclusive observational studies across health care databases. The sample in this case was a study cohort (N= 123,712) of patients who had been admitted to a hospital for unipolar depression between January 1, 1987 and December 31, 2012. They had a comparison incident cohort (N=30004) that looked at new patients beginning on December 31, 1996 with no mental health diagnosis, hospital admissions for depression, exposure to the medications of interest, or history of outpatient care in the year prior to the start. The purpose of the incident cohort was to look at the issue of survival bias. The primary outcome measure was risk of readmission in all patients admitted for unipolar depression at least once between 1987 and 2012. All cause admissions were a secondary outcome measure. Medication use was estimated using the PRE2DUP mathematical modelling of the patient medication purchasing. Each patient was used as their own control to eliminate selection bias. I did not have access to the appendices from this study, so I will forgo further discussion of the statistical methodology without that data.
Mean hospitalizations for people who had used lithium was 4.3 (SD 6.9) for psychiatric admission and 7.8 (SD 9.1) for all cause admissions compared to 2.2(SD 3.1) and 5.8(SD 7.0) for those who had not indicating the lithium treated patient were more severely ill. Using lithium significantly decreased the relapse risk. Mean daily lithium dose was noted to be 765 mg. Forest plots are contained in the body of the article looking at the hazard rations of readmission for several pharmacological treatments in both the study cohort and the incident cohort. Forest plots looked at benzodiazepines, hypnotics, antipsychotics, and lithium +/- antidepressants.
Lithium monotherapy was superior to all other studied pharmacotherapies in preventing hospital readmissions. Older antidepressants (amitriptyline and doxepin) and antipsychotics (clozapine sulpride, aripiprazole, and quetiapine) showed some efficacy in preventing hosptializations. Benzodiazepines did not. In the incident cohort where (survival bias was eliminated) the lithium effect on decreased rehospitalizations was more robust suggesting the effect size was more valid than for the total cohort. They also did a secondary mortality assessment and showed that both lithium and antidepressants were associated with decreased overall mortality and no difference was observed for antipsychotics.
The authors recognize that there are significant adverse effects and limitations with the use of lithium. They point out there are also possible advantages outside the treatment of mood disorders but there is a significant burden associated with taking it. They recommend that is be considered for maintenance of a broader group of patients with unipolar depression. In our antidepressant-centric society, I agree with their conclusion. The effects in this study really cannot be ignored. Lithium augmentation of antidepressants has been around for over 30 years. It surfaced again the the STAR*D protocol. I see patients who have been exclusively been treated by other physicians or prescribers and in the past 8 years I have seen exactly 1 patient with unipolar depression who was being treated with lithium augmentation. It is far more common to see patient taking 2-4 antidepressants (typically SSRI + bupropion + trazodone or mirtazapine) or an antidepressant plus lamotrigine as the augmentation strategy. A similar study with a cohort of American patients would be very useful to look at questions about the efficacy of lithium versus the antidepressant polypharmacy or lamotrigine - but my concern would be that there would not be a large enough sample of patients taking lithium.
It may be up to clinicians who are used to lithium therapy and the application to unipolar depression to reintroduce the practice and collect data on what the outcomes are relative to standard antidepressant augmentation strategies. Overall this study from a group of pharmacoepidemiologists provides compelling data to take a second look at an old strategy instead of just adding more antidepressants. The data looks so good, lithium is so inexpensive, and in the USA we are in an absolute vacuum of lithium non-use for unipolar depression. Inpatient treatment for depression has become so atrocious that it has never been more important to help people stay out of the hospital. That is why I considered this to be an important paper.
George Dawson, MD, DFAPA
References:
1: Goodwin, FK, Jamison, KR. Manic-depressive illness. New York: Oxford University Press, 1990: pp.693-696.
2: Tiihonen J, Tanskanen A, Hoti F, Vattulainen P, Taipale H, Mehtälä J,Lähteenvuo M. Pharmacological treatments and risk of readmission to hospital for unipolar depression in Finland: a nationwide cohort study. Lancet Psychiatry. 2017 Jul;4(7):547-553. doi: 10.1016/S2215-0366(17)30134-7. Epub 2017 Jun 1. PubMed PMID: 28578901.
3: Prien RF, Klett CJ, Caffey EM Jr. Lithium carbonate and imipramine inprevention of affective episodes. A comparison in recurrent affective illness. Arch Gen Psychiatry. 1973 Sep;29(3):420-5. PubMed PMID: 4579507.
4: Prien RF, Caffey EM Jr, Klett CJ. Prophylactic efficacy of lithium carbonate in manic-depressive illness. Report of the Veterans Administration and National Institute of Mental Health collaborative study group. Arch Gen Psychiatry. 1973 Mar;28(3):337-41. PubMed PMID: 4569674.
7: Bauer M, Bschor T, Kunz D, Berghöfer A, Ströhle A, Müller-Oerlinghausen B.Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000 Sep;157(9):1429-35. PubMed PMID: 10964859.
Supplementary:
Figure 1 above is directly from reference 2 with permission from Elsevier. Licensing agreement #4270040486127.
Friday, January 5, 2018
If Your Patient Really Needs Lithium.......
I had the good fortune to work in an intense medical setting for about 22 years where I was responsible for the medical care of my patients. In that setting I had access to excellent specialists, in fact some of the finest physicians I have seen anywhere. They were typically involved when I had identified a medical problem that potentially complicated the psychiatric care of my patients. In those cases - no matter what the problem was it usually came down to the question: "If your patient really needs this medication we need to continue it." One of the most significant complications was renal failure of varying degrees while taking lithium. Some patients on my unit were hospitalized because they had been stable on lithium but were developing renal failure and needed to be tried on another agent. In some cases they experienced severe associated complications including delirium either from the bipolar disorder or medications used in the transition. In some cases, they needed dialysis and renal medicine consultants would advise me on the lithium dose to try while they were receiving hemodialysis. All of this experience led me to have a very low threshold for recognizing and acting on potential adverse effects of lithium as early as possible.
At various points in my career, there was a question of renal failure occurred in cohorts on lithium or it was due to a different cause. As an example, the Lithium Encyclopedia from 1983 (1) stated concisely: "There have been a growing number of reports of morphological and functional kidney damage associated with lithium use. The actual incidence of lithium nephrotoxicity is not known. Most researchers believe that irreversible damage is not widespread but that risk increases with length of treatment and serum lithium level."
At the time there were opinions that up to 20% of patients on long term lithium maintenance had morphological changes and functional changes primarily with water absorption but no reduced glomerular filtration rate (GFR) or renal insufficiency. My favorite renal and electrolyte disorders text cited lithium as a compound that caused vasopressin resistant nephrogenic diabetes insipidus (NDI) (2). More recent evidence suggests that 15-20% of patients on lithium develop a progressive decrease in GFR typically does not progress to end stage renal disease (ESRD) and dialysis but in some cases it clearly does. These generalizations are usually based on low numbers of patients who have been identified as having a specific creatinine or GFR. A creatinine of 2.5 mg/dl predicted progression to ESRD in most patients. A larger study in Sweden of 3369 patients, suggested a lower threshold. In that study, 13 patients had been off lithium for 2 years before starting dialysis and 11 had a creatinine of > 1.4. Some were lower but there had been a progressive increase from baseline suggesting the rate of change is important. Additional factors such as the use of NSAIDS and ACE inhibitors like lisinopril as well as other intrinsic kidney diseases can be a factor.
There have been a recent increase in studies that look at the side effects of lithium and in one case (6) compare lithium side effects to other typical mood stabilizers (valproate, olanzapine, questiapine). One of the studies uses descriptive statistics and the other two involve calculations of hazard ratios for specific risks. The results are fairly uniform in their conclusions and have been known in most psychiatry training programs for the past 10-20 years. The articles here use the Kidney Disease Outcomes Quality Initiative (KDOQI) staging of chronic kidney disease published in 2002. The range is from stage 1 (eGFR ≥ 90 ml/min/1.73 m2 ) to stage 5 (eGFR < 15 ml/min/1.73 m2 or dialysis). For the purpose of these papers Stage 2 is an eGFR of 60-89 and Stage 3 is 30-59. As previously noted progression can occur in some cases even years after the lithium has been stopped.
The most interesting of the three papers is probably from Hayes, et al because of their strategy to look at lithium toxicity but in the context of other commonly used mood stabilizers (valproate, olanzapine, quetiapine) and the associated toxicities of these other mood stabilizers. As a result they have a table entitled Table 2. Adverse effects during maintenance treatment that looks at these mood stabilizers and CKD (stage 3 and 4), hypothyroidism, hyperthyroidism, hypercalcemia, Type 2 diabetes mellitus, cardiovascular disease, hypertension, hepatotoxicity, and weight gain (greater than 7% and greater than 15%). Hazard ratios care calculated for all of these adverse effects using lithium as the reference. The number of events in each category is relatively low compared with the total events in a large clinical sample. The adverse events described were expected. They estimated the rate of severe CKD (stage 4 or 5) as 1 in 100 person years at risk. The estimated rates of stage 3 and stage 4 CKD were 9 in 100 per years at risk. Hepatotoxicity was rare in the sample and was elevated in the quetiapine group. Weight gain was most significant for olanzapine, quetiapine, and valproate relative to lithium. Olanzapine also had the highest rate of new onset hypertension.
None of this information deters me from offering lithium to people who I think are good candidates. The rationale is that lithium can be a life changing medication, especially for people who have generally never achieved mood stabilization with all of the typical medications prescribed for mood disorders these days. Informed consent should include this potential complication. I generally advise people that there is a 40% risk of NDI of varying severity, the need for ongoing testing, the need to avoid additional medications that may complicate the use of lithium, and finally close self monitoring of both side effects and any situation that can complicate lithium therapy like conditions leading to dehydration. I will add the risk of mild CKD and explain to the patient what that means.
One of the interesting aspects of these articles is that ongoing screening for patients on lithium therapy varies considerably from setting to setting. National guidelines seem to help. In these large scale studies there is not enough discussion of work at the clinical level incorporating the red flags from the research. For example it is obviously important to know about new medications, especially ACEIs, NSAIDs, and serotonergic medications. Medications that may complicate interpretation of lab finding like Vitamin D and Calcium supplementation in the case of hypercalcemia are also important. The review of systems is also important anywhere along the spectrum of side effects including neurological, cognitive, gastrointestinal, and renal symptoms. I generally advise patients that increasing nocturia, polyuria, and polydipsia with associated laboratory finding increases the risk of irreversible renal changes including a low risk of progression to ESRD. The presence of edema may indicate the rare onset of a nephrotic syndrome that can occur is some people with acute treatment.
The good news is that even now there is an accumulating literature on the potential complications of lithium therapy and some general ideas about what psychiatrists should do about it. Although the methodology of these studies varies significantly they generally come to similar conclusions about the effect of lithium on renal function, thyroid function, and calcium metabolism. All of these systems require close monitoring by assessing the patients clinical status and lab testing. Numerous changes in clinical status should result in departures from any monitoring routine. The critical elements is communication with the patient and education about when the psychiatrist should be contacted.
George Dawson, MD, DFAPA
References:
1: Jefferson JW, Greist JH, Ackerman DL. Lithium Encyclopedia for Clinical Practice. American Psychiatric Press, Inc. 1983: p151.
2: Schrier RW (ed). Renal and Electrolyte Disorders, 2nd Ed. Little, Brown and Company, Inc. Boston 1980: p. 31.
3: Lerma VE. Renal toxicity of lithium. In UpToDate. Sterns RH, Sheridan AM (eds) (Accessed on January 2, 2018).
4: Bendz H, Schön S, Attman PO, Aurell M. Renal failure occurs in chronic lithiumtreatment but is uncommon. Kidney Int. 2010 Feb;77(3):219-24. doi: 10.1038/ki.2009.433. Epub 2009 Nov 25. PubMed PMID: 19940841. (see open access text).
5: Dineen R, Bogdanet D, Thompson D, Thompson CJ, Behan LA, McKay AP, Boran G, Wall C, Gibney J, O’Keane VO, Sherlock M. Endocrinopathies and renal outcomes in lithium therapy: impact of lithium toxicity. QJM: An International Journal of Medicine, Volume 110, Issue 12, 1 December 2017, Pages 821–827, https://doi.org/10.1093/qjmed/hcx171
6: Hayes JF, Marston L, Walters K, Geddes JR, King M, Osborn DP. Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study. PLoS Med. 2016 Aug 2;13(8):e1002058. doi: 10.1371/journal.pmed.1002058. eCollection 2016 Aug. PubMed PMID: 27483368.
7: Shine B, McKnight RF, Leaver L, Geddes JR. Long-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet. 2015 Aug 1;386(9992):461-8. doi: 10.1016/S0140-6736(14)61842-0. Epub 2015 May 20. PubMed PMID: 26003379.
Saturday, December 30, 2017
The Nocebo Effect In Antidepressant Trials
Early in my career I was an assistant to principle investigators who were doing clinical trials of drugs to treat generalized anxiety disorder, major depression, schizophrenia, and Alzheimer's Disease. My job was top screen patients, do medical histories and physical examination, and follow them throughout the research protocol. That is not an easy job. The difficult part is making sure that the research patients are not getting any medical complications from a new and experimental drug in addition to assessing any possible therapeutic effects.
One of the main complications was the nocebo effect. With all of the hype about placebo effects with antidepressants, I have always found it curious that few mention the nocebo and how it affects clinical research. In those early days it was possible to break the blind and if research subjects withdrew from the protocol to let them know if they were taking active drug or placebo. An adverse reaction to placebo is the nocebo effect. It was common in these protocols for research subjects to either stop the protocol due to adverse effects or describe severe side effects while they were taking placebo.
One of the main problems I encountered with this effect in clinical trials that is an even bigger problem today is loss of research subjects who terminated early due to nocebo effects. In the example I gave the patients developed significant side effects in response to a placebo, but people can also develop dramatic side effects to the active research medication. Whether they are more likely to get the effect from active drug or an active placebo over the active study drug has not been actively studied. These are critical questions because FDA trials now require and Intent To Treat Analysis for clinical trials. That means all of the research subjects who did not complete the protocol for whatever reason need to be included in the analysis. That becomes a problem when any subject has not been treated with the active drug - both in terms of true response but also side effects reporting. All FDA package inserts contain detailed information on medication side effects. Today in many cases there is a side effect comparison between active drug and placebo. Nocebo related data can skew the side effect data reported in the package inserts.
I always thought that antidepressant trials were the ideal setting to study nocebo effects and came across a paper two years ago that does that (1). That time frame over the last two years was an interesting one. We saw all of the speculations about the release of the DSM-5 in the summer of 2015. Much of that speculation involved sensational articles in the media suggesting that antidepressant medications did not work or ate least were no better than the placebo effect. Further speculation suggested that pharmaceutical manufacturers and the American Psychiatric Association (via the DSM-5) had an interest in broadening the market for antidepressants and increasing their use. At no point did any of the critics suggest that their may be a serious problem with clinical trials based on the nocebo effect. And most interestingly, many of the critics in non-professional forum complained mostly about the side effects of these medications. In some cases they described these medications as very dangerous.
The paper in reference 1 looks only at people receiving placebo in a pooled sample of placebo-arm data from 20 industry-sponsored multi-site randomized clinical trials of antidepressant medication in the treatment of acute major depression. Adverse event data was recorded for all 20 clinical trials. Adverse events (AE) can be pre-existing nonspecific somatic symptoms. Treatment emergent adverse events (TEAE) were events that occurred or worsened during placebo treatment. The adverse events were obtained by open ended questioning and in clinical trials from that period (1993-2010) were listed on standard forms. Five endpoints were studied including any AE, any treatment related AE, any severe AE, any serious AE resulting in discontinuation from the study, and discontinuation form the study for any reason. Worsening of clinical symptoms was also measured and defined as any increase in the depression rating score on any scale used for that particular study.
The main results included:
1. TEAEs from placebo were reported in 1,569/2,457 or 63.9% of the study participants.
2. 11.2% (274) of the participants had worsening depression rating scores during the placebo treatment.
3. 4.7% (115) of the participants discontinued the study due to an AE during the placebo treatment.
Just considering those results and nothing more illustrates the nocebo problem with current state-of-the-art clinical trials technology. If 63.9% of the placebo-treated subjects experience side effects - what does that translate to in terms of subjects taking the active drug getting side effects unrelated to that drug? Although untreated depression might be expected to worsen - how much of that is nocebo modulated and how much crosses over to the active drug. A significant number of people dropped out of the trial due to the nocebo effect. The main results suggest that in randomized placebo controlled trials of efficacy and safety - the nocebo effect is substantial. Since the observed TEAEs and percentage of research subjects worsening on placebo was substantial - extension to TEAEs, worsening, and dropping out in clinical practice could be expected. That would be a very difficult problem to research due to a lack of standardization across practices.
The authors did not stop with those results. They looked at additional variable to see if there was any evidence to confirm either of the two major hypotheses about the nocebo effect. The first is a conditioning hypothesis. In this effect, prior exposure results in the expectation of an associated effect. They cite as an example, women receiving chemotherapy for breast cancer and how an associated stimulus led to more nausea in the conditioned group (4). In this case they looked at previous treatment with antidepressants as a possible conditioning effect and did not find any significant associations.
The other major hypothesis regard the nocebo effect is an expectation hypothesis. They cite an example where a sample of college students were given inert placebo and told that it was an herbal supplement for cognitive enhancement (5). They were provided with a fictitious list of potential benefits and side effects. Symptoms were endorsed by most students but the students who believed that they received the active supplement endorsed more symptoms suggesting that their expectation of supplement and effect affected their perception. They found some support that previous treatment with Hypericum perforatum (St. John's Wort) may have been associated with a greater likelihood of reporting TEAEs and suggest that users of complementary medications may be more suspect of medical pharmacotherapy.
The paper is a fairly concise review of demographic and neurobiological factors associated with the nocebo effect. On the neurobiological side they cite the work of Enck, et al (6). Elman and Borsook (7) have an interesting paper that looks at addiction, pain, and several common substrates of the placebo and nocebo effects.
The take home message for me is what I have known for over a decade at this point. Current clinical trials technology in psychiatry and medicine is general is very primitive. The evidence-based movement has had its day if this is the kind of evidence they are considering. Why would anyone expect much more than a mild to moderate effect from a medication used for heterogeneous disorders when the true effect of the medication is significantly affected by two factors that are never measured? This suggests areas for improvements in clinical trials that could render much of what has been collected so far - obsolete.
A final observation comes to mind and that is a phenomenon that I wrote about on this blog many years ago. People with little to no expertise in psychiatry or medicine don't hesitate to criticize the field. A good example would be people who have never treated a single case of depression much less thousands of cases who do a meta-analysis and claim that antidepressants are placebos. I don't recall any of these critics in the popular press considering the limitations of clinical trials. Without that basic consideration any theory that fits the apparent data can apply.
What would be useful at this point would be detailed analyses of the placebo arm of all clinical trials and a discussion of how these effects might bias the data. Actual interventions to quantify or eliminate these effects in future trials is the next step. These are more practical steps than hoping for mythical large clinical trials with slightly more sophisticated clinical trials technology that epidemiologists and the Cochrane Collaboration routinely recommend.
George Dawson, MD, DFAPA
References:
1: Dodd S, Schacht A, Kelin K, Dueñas H, Reed VA, Williams LJ, Quirk FH, Malhi GS, Berk M. Nocebo effects in the treatment of major depression: results from an individual study participant-level meta-analysis of the placebo arm of duloxetine clinical trials. J Clin Psychiatry. 2015 Jun;76(6):702-11. doi: 10.4088/JCP.13r08858. PubMed PMID: 26132671.
2: Gupta SK. Intention-to-treat concept: A review. Perspectives in Clinical Research. 2011;2(3):109-112. doi:10.4103/2229-3485.83221.
3: Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia” by A. Tinnermann, S. Geuter, C. Sprenger, J. Finsterbusch, C. Büchel in Science. Published online October 5 2017 doi:10.1126/science.aan122
4: Bovbjerg DH, Redd WH, Jacobsen PB, Manne SL, Taylor KL, Surbone A, Crown JP,Norton L, Gilewski TA, Hudis CA, et al. An experimental analysis of classically conditioned nausea during cancer chemotherapy. Psychosom Med. 1992 Nov-Dec;54(6):623-37. PubMed PMID: 1454956.
7: Elman I, Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 2016 Jan 6;89(1):11-36. doi: 10.1016/j.neuron.2015.11.027. Review. PubMed PMID: 26748087.
Attributions:
Figure at the top is stock photo from Shutterstock per their licensing agreement by kasezo entitled:
Stock illustration ID: 284558927 conceptual 3d design of false pill.( placebo and nocebo effect.red and green colored version)
Friday, December 29, 2017
Language and the Nocebo Effect
Arthur Barsky wrote an article in this week's JAMA entitled "The Iatrogenic Potential of the Physician's Words." Dr. Barsky is one of those authors I have been reading over the past 20 years. He is a psychiatrist and an expert on psychosomatic medicine. Like a number of authors, when I see his name - I just read the article. It is automatic. This article is brief but it contains a lot of wisdom about how communication with patients can affect the expected outcome of medical treatment. There are several points in the article that just jump out at you if you are an experienced clinician and have followed many patients.
The single most important sentence in the article starts the second paragraph:
"Somatic symptoms and underlying disease do not have a fixed, invariable, one-to-one equivalence." Physicians of course are trained to see a multitude of presentations of illness. Influenza is a good example. The majority of people are more ill than if they have the common cold, but a significant number are critically ill and dying. For example in 2016, the CDC estimated that about 24.6 million people got the flu, 308,000 were hospitalized, and 11,995 died. Theoretically there are a number of people with few or no symptoms who can still spread the virus. There are a significant number of factors in both the disease vector and the host that determine a wide array of outcomes. Infectious diseases provide the most straightforward example or variability in symptoms, underlying illnesses and outcome.
With the chronic complex, polygenic illnesses that require ongoing medical care the situation is less clear. In this case we have a patient who is constantly updating their assessment of their problems, symptoms, and state of their illness. They make similar determinations about any medicine or treatment that their physician wants them to take. A significant part of that determination is based on the information that they obtain from their physician or other sources and more importantly how it is presented. From a psychiatric perspective it should be presented as neutral as possible, but patients vary greatly in their reactions to even fairly dry information. As an example, I typically provide patients with MedlinePlus information on their medications. I consider these documents to present all of the essential unbiased information. It is still common to encounter people who tell me: "Don't give me that doctor! I know if I read it I will get every side effect that is listed!" I honor their request and reassure them that they can call me if there are any problems.
Dr. Barsky focuses on the informed consent aspect of treatment and how the information presented can affect the patients reaction to the treatment. He gives examples of men warned about the sexual side effects of beta blockers getting roughly twice as much sexual dysfunction and back pain patients undergoing spine imaging having more pain, functional impairment and physician visits. Psychiatry has the additional burden of many more vocal critics promoting the side effect burden of medications, often to the point that it seems like there was a conspiracy to "cover them up". That results in patients in the office complaining about what they have read online, even before the informed consent discussion begins.
There is a discussion of the nocebo effect or adverse effects to placebo treatment that does not receive enough discussion within medicine. I had early familiarity with this phenomenon from my work with clinical trials of pharmaceuticals (antidepressants, anxiolytics, antipsychotics). In each case the active drug was compared to placebo and patients were followed closely. In those days we were allowed to break the blind to tell patients if they were on active drug or placebo. The first time I encountered the nocebo effect, I was treating a young man with an experimental anxiety medication. He bitterly complained of many side effects initially and at the second or third visit said that he could no longer stand it. He needed to be taken off the medication because it was making him extremely ill. I broke the blind and told him that he was taking an inert placebo. He was somewhat embarrassed but the symptoms resolved during the time he was sitting in my office. Since then I have seen the nocebo effect hundreds of time in clinical practice. Many of the predisposed claim a high sensitivity or allergy to multiple medications and warn me ahead of time that they need to start on the lowest possible dose of medication. Even then it is dicey.
Over the years that led me to develop a uniform informed consent decision designed to minimize the nocebo effect. I think it is fairly straightforward to provide all of the relevant information in a manner that transmits both the side effect but also the likelihood of developing the side effect to the patient. Worst case scenarios are never avoided. For example, I always warn people on serotonergic medications about the risk of serotonin syndrome and the fact that it can be lethal. I also let them know that the chances of developing serotonin syndrome are very low. I use the same approach for tardive dyskinesia and other tardive syndromes, diabetes mellitus, cardiac arrhythmias, metabolic syndrome, seizures, neutropenia and agranulocytosis, and neuroleptic malignant syndrome. But also give them an estimate of how likely it is that they will develop that side effect or how often I have seen the side effect in 30 years of practice. That approach seems to have the expected effect on false positive reports of these symptoms and in fact I am much more likely to notice threshold symptoms (hypophonia, micrographia, etc) than the people who I warn about the side effects.
A second tier of symptoms surrounds the issues of sexual side effects and weight gain. A significant number of people are averse to trying any medication that might result in either side effect. That can occur even when they want to take the medication. In that situation it is useful to have a comprehensive reference that described the entire range of side effects in clinical trials for specific medications and present the facts. The facts will generally illustrate that the side effect of concern is not universal by any means and can be addressed if it happens in the case of sexual side effects or proactively (at least in some cases) in the case of increased appetite and weight gain.
Given the long list of potential side effects, reassurance that they will be assessed and a plan put in place to address them is the best approach to informed consent. The other discussion that most people find reassuring is that I have a low threshold for stopping a medication that causes any side effect that a patient finds distressing. During residency I had the experience of stopping an antidepressant medication that a patient had been on for 5 years. He was complaining primarily of a flu-like syndrome and headaches. After that medication was stopped those symptoms cleared up entirely. Since that incident, I routinely advice patients that I don't advise that they try to "get used to" medications and that I would prefer we just stop a medication if it is causing problems and try a different approach where possible.
Barsky invokes viscerosomatic amplification as a likely cause of information affecting symptom perception. He provides a very interesting supplement outlining a process whereby both benign but noticeable bodily sensations and disease symptoms of varying intensity are biased by information provided by the physician leading to a feedback loop of increasing anxiety, vigilance, and reactivity. Psychiatrists used to treating anxiety disorders are probably very familiar with this mechanism. I routinely ask those patients if they have cardiac awareness. Are they aware of their heart beating as they lay awake in bed at night waiting to fall asleep? Is it more intense than that - like their entire body is pulsating? What is their theory of what is happening? Are they concerned that there is something wrong with their heart? That phenomenon suggests that anxiety plays a significant role in selective attention and hypervigilance that is internally focused. It also suggests a major role for temperament and personality factors in the hypervigilance process since so many patients experience the anxiety at that level.
Concrete steps to minimize the symptom amplification process are discussed and they logically follow the proposed mechanism. The meaning to the patient of the symptoms needs to be explored. Education can be provided about both the nocebo effect and the amplification process. I typically use my research vignette for the nocebo discussion. The purported evolutionary aspects of anxiety and how it results in theories including somatic theories is a good starting point. The article suggests that "contextualized informed consent" can be useful or a method not enumerating "benign, nonspecific symptoms" with the patient's consent. This is often a practical matter given the length of rare and nonspecific symptoms included in package inserts these days. If the patient present with what I consider to be a rare side effect, I will look it up during the session, give them a ballpark estimate of how common it was in the clinical trials and discuss other potential causes.
The article also points out that side effects can also point to problems in the physician-patient relationship. Psychiatrists who are training in therapeutic neutrality often encounter difficulties with dependent patients who frequently present as not tolerating any medication or suggestion.
In addition to typical prescription scenarios this dynamic applies to other important clinical scenarios. Treating acute and chronic pain is a case in point. There is probably no better example of patient expectations based on physician advice than this prescribing relationship. If opioids are involved the scenario is complicated by a medication that reinforces its own use. The physician discussing medication response can bias the patient in the direction of dose escalation in the context of what is perceived as an incomplete response to pain when chronic pain does not respond completely to opioid analgesia.
In many cases entire illnesses are the result of problematic information supplied by physicians. Cases of chronic Lyme disease and the extremes that many of these patients go to including the application of devices that constitute medical quackery is another example. The best approach in these cases is to discuss the patient's theory of their current symptoms and how anxiety amplifies these symptoms. At that point an alternate more plausible theory for their distress can be discussed and it often results in significant improvement if not resolution of symptoms.
I can't recommend this brief article by Dr. Barsky enough. It should be read and understood by all psychiatric trainees and practicing psychiatrists. These concepts are critical to both discussions with patients about a treatment plan in order to avoid iatrogenic effects and to make sense out of somatization.
George Dawson, MD, DFAPA
Reference:
1: Barsky AJ. The Iatrogenic Potential of the Physician's Words. JAMA. 2017 Dec 26;318(24):2425-2426. doi: 10.1001/jama.2017.16216. PubMed PMID: 29090307.
Attributions:
Figure at the top is stock photo from Shutterstock per their licensing agreement by kasezo entitled:
Stock illustration ID: 284558927 conceptual 3d design of false pill.( placebo and nocebo effect.red and green colored version)
Sunday, December 17, 2017
Less Is Less - A World of Difference Between Psychiatry and Cardiology
I read Lisa Rosenbaum's opinion piece in this week's New England Journal of Medicine (1). She discusses both sides of the rationing coin. On the one hand, we don't want to reduce resources to the point that people do not get necessary care. On the other hand there are forces including financial incentives and the inability of physicians to tolerate the diagnostic uncertainty of not performing the necessary tests that lead to both increased cost and in some cases unnecessary risk to the patient. She provides an example from her personal medical history on forgoing a recommended test with no associated adverse outcome. A lot of the article is written from her perspective as a cardiologist or cardiology fellow. I can recall, the think tank studies from the 1980s suggesting that coronary artery bypass surgery was overutilized. There are many studies that suggest that medical treatment of coronary artery disease provides similar outcomes. Today we hear the same arguments about the treatment of the atrial fibrillation epidemic and the equivalence of rate control versus rhythm control. The options are presented as a coin toss to many patients. But in both cases it is much more than that. Anytime population based averages of care are applied across large populations there will be a significant number of people who do worse than the norm and may have done better with the other option. My concern has always been, the implicit pressure by healthcare companies to make money by exerting pressure in the direction of the least expensive option right up to including no care at all.
Dr. Rosenbaum discusses the "less is more" movement and the Choosing Wisely campaigns to reduce unnecessary care. She discusses the early role of the Dartmouth Atlas in pointing out the lack of correlation between cost of care and outcomes - a notion that has been discredited (2) but it was the mantra of administrators for nearly two decades. She concludes that these movements resulted in the idea that "less care is better care or that more care is harmful." She reviews more recent data that higher spending is associated with better outcomes. She includes recent research on unnecessary admissions and how Medicare beneficiaries discharge from the emergency departments (ED) of hospitals with the lowest admission rates were 3.4 times as likely to die with a week than similar patients admitted to hospitals with higher admission rates - even though those same patients were healthier.
She discusses overdiagnosis in cardiology. Unlike psychiatry, cardiology has a considerable array of biochemical markers, electrophysiological studies, and imaging studies that are very useful in the diagnosis and management of their patients. She illustrates the trade offs involved in considering false positives for troponin and how liberalization of the cut-off values leads to better diagnosis and treatment rather than overdiagnosis. In the area where I currently practice, the entire landscape for diagnosing and treating suspected acute coronary syndrome (ACS) has changed significantly. Nobody tries to guess if chest pain has a cardiac origin or not. Middle-aged patients are generally admitted and tested with troponin levels and an exercise stress test the following morning if the troponins are negative. If the stress test is negative they are sent home. In most acute care metropolitan hospitals there is ample intensive care and telemetry space to accommodate all of these admissions. The cost of that overnight admission to cardiology exceeds the cost of a week long admission to an inpatient psychiatric unit with a psychosis diagnosis.
What is the parallel process on psychiatry? A patient in crisis presenting to an ED of a metro hospital in crisis has no similar guarantee of cautious screening. In the majority of cases they will never see a psychiatrist. In most cases the assessment and screening is done by nonphysicians. In addition, diagnoses and syndromes are generally secondary in the discharge process. The only way that patient gets admitted is dangerousness to self or others. That could be due to an acute intoxication, an emotional overreaction, a mood disorder, a developmental disorder, a neurodegenerative disorder, or a psychosis. The only thing that counts is the dangerousness. There are no biochemical markers or imaging markers of dangerousness. There is significant disagreement in many cases among clinicians, patients, and their families. If a person is admitted either voluntarily or on a legal hold - in any case they will typically find themselves sitting on a psychiatric unit until somebody determines that they are no longer dangerous. Hopefully they will see a psychiatrist and other skilled professionals like trained psychiatric nurses, social workers, and occupational therapists - but there is no guarantee. The issue in an acute dangerousness based psychiatric hospitalization is not a question of overdiagnosis - but whether the patient will get the correct diagnosis and an adequate medical evaluation and discharge plan. The driving force for that is rationing. The cost of an overnight stay on a cardiology unit with telemetry, blood tests, and an exercise stress test in the morning easily exceeds the payment for complex psychiatric care. I would say that complex psychiatric care is the equivalent to treating a person with a psychosis, extreme mania, or life threatening catatonia or depression. In general we are not worried about the issue of overdiagnosis. People flee psychiatric units if they are given the opportunity and they don't really care if they get diagnosed or not. Psychiatrists cannot present them with an array of options because there aren't any.
When I saw the term overmedicalizing in Dr. Rosenbaum's title - I wondered if she was aware of its Szaszian origins? Szasz was apparently so enthralled by a form of psychiatric treatment that was totally subjective and more akin to a literary critique that he suggested society has an interest in using psychiatry as a way to exert social control over certain subgroups. The logical conclusion is that mental illness is not a disease and calling something an illness is strictly a power play. For some reason society and its unholy alliance with psychiatrists is seeking to exert power over a subset of society for unclear reasons. I doubt that Dr. Rosenbaum is using the Szaszian definition. She is probably referring to any number of situations where non-disease is treated as disease.
There are many problems with Szasz - not the least of which is how he would end up treating any number of severe mental conditions. More modern authors on what is and is not a disease seem confused about the imprecise definition, especially in the absence of gross pathology. There is no family member affected by schizophrenia, bipolar disorder, depression, alcoholism, or addiction that doubts for a moment that these are diseases. They generally don't doubt that psychiatrists, at least until very recently were the physicians most interested in treating these problems.
Dr. Rosenbaum's theme does not seem to apply to psychiatric practice. There are no expensive tests to overutilize. Stays on inpatient units are capped by ridiculously short lengths of stay that do not reflect the severity of illness. Even then - admissions to psychiatric units are generally under the control of emergency physicians. This is part of the oversimplication, that she referred to. That oversimplification characterized all inpatient stays by diagnosis related groups (DRGs) and suggested that all inpatient stays could be kept to a certain number of days or cost. Nothing else was necessary. This led to three outcomes that led to very subpar care. The first outcome was the deterioration of inpatient services. Rationing does not maximize state of the art care and practically all inpatient units are essentially observation services waiting for people to become less dangerous. Dialogue with patients on acute care units is essentially focused on that issue. Addressing the psychiatric disorder is a consideration only as it applies to dangerousness. I have had many utilization reviewers tell me that they would no longer pay for the treatment of extremely ill people because they did not seem to be dangerous.
The second outcome was splitting off addiction treatment. At some point, a large number of detox admissions were directed to psychiatry because medicine units no longer did detoxiification. Then at some point to capitalize on the DRG payments, psychiatric units not longer did detox. patients with addiction were sent from the ED to a county detox unit. The only time they came back is if they experienced seizures or delirium tremens. The overall rationale is saving the insurance companies money. They don't cover people at county detox units.
The third outcome is that patients with severe psychiatric disorders are sent to jail rather than inpatient units. This has resulted in county jails becoming the largest psychiatric hospitals in the United States at a time when psychiatric beds per capita here are among the lowest in the world according to OECD data. All of these changes are associated with a tremendous lack of quality and would be a national embarrassment - if they were not viewed as cost effective by the businesses and governments in charge. The American Psychiatric Association and other district branches still incorporate the cost effective rhetoric when in fact, psychiatry left cost effective in the rear view mirror thirty years ago.
Psychiatrists don't have expensive procedures to order. In psychiatry less is less (or no) time seeing a psychiatrist. Less is no time being treated in a medically supervised and therapeutic inpatient or detox unit when you need it. Less is no psychotherapy that might work for you. Less is no case management services. Less is no public health nursing. Less is not taking the best medication because a pharmaceutical benefit manager says you will have to pay full price for it. Less in no available child psychiatrist when they are needed. Less is not getting your blood pressure checked in a public clinic because there are no blood pressure cuffs.
Less in psychiatry is obviously far less than any other speciality.
That brings me to the last concept in the article illusions of value. There is no greater illusion of value than current psychiatric care and that is not because of psychiatrists. To give a clear example, I am an excellent diagnostician - both medical and psychiatric illnesses. I can figure out what is wrong with people and come up with a plan to address all of those issues. I can't do it in a 15 minute appointment. I can't do it if I have to type up all of my encounters like a stenographer or waste my time supporting horrible electronic health record software. In the case of people with severe problems, I can't do it without staff people who can get the patient to the appointment to see me and make sure that the person follows up with all of my recommendations. Without all of that infrastructure on the outpatient side, I will end up seeing about 60% of the people who are scheduled and the average person coming back will tell me they are taking their prescribed medication half of the time. Almost all of that supporting infrastructure has been eliminated in the past 30 years and managed care organizations have set up psychiatric services based on the prescription of a medication. Even if you have a severe problem. Show up 3 or 4 times a year, have the psychiatrist ask you a few questions, and get enough refills until the next appointment.
Psychiatry is actually a paradigm that the rest of medicine should look to in terms of less is less. In her final sentence Rosenbaum describes "less is more" as an aphorism that is "better suited to telling coherent stories than to the complex decisions faced by doctors and patients." I could not agree more. My only qualifier would be that the administrators are always telling their coherent stories that make it seem like they know more than physicians know about medical practice. They do a great job of selling it and convincing people that a symptom checklist and an antidepressant prescription constitutes optimal care.
That is the only way that the current abysmal psychiatric services offered by large health care corporations could get a pass.
George Dawson, MD, DFAPA
References:
1: Rosenbaum L. The Less-Is-More Crusade - Are We Overmedicalizing or Oversimplifying? N Engl J Med. 2017 Dec 14;377(24):2392-2397. doi: 10.1056/NEJMms1713248. PubMed PMID: 29236644.
That brings me to the last concept in the article illusions of value. There is no greater illusion of value than current psychiatric care and that is not because of psychiatrists. To give a clear example, I am an excellent diagnostician - both medical and psychiatric illnesses. I can figure out what is wrong with people and come up with a plan to address all of those issues. I can't do it in a 15 minute appointment. I can't do it if I have to type up all of my encounters like a stenographer or waste my time supporting horrible electronic health record software. In the case of people with severe problems, I can't do it without staff people who can get the patient to the appointment to see me and make sure that the person follows up with all of my recommendations. Without all of that infrastructure on the outpatient side, I will end up seeing about 60% of the people who are scheduled and the average person coming back will tell me they are taking their prescribed medication half of the time. Almost all of that supporting infrastructure has been eliminated in the past 30 years and managed care organizations have set up psychiatric services based on the prescription of a medication. Even if you have a severe problem. Show up 3 or 4 times a year, have the psychiatrist ask you a few questions, and get enough refills until the next appointment.
Psychiatry is actually a paradigm that the rest of medicine should look to in terms of less is less. In her final sentence Rosenbaum describes "less is more" as an aphorism that is "better suited to telling coherent stories than to the complex decisions faced by doctors and patients." I could not agree more. My only qualifier would be that the administrators are always telling their coherent stories that make it seem like they know more than physicians know about medical practice. They do a great job of selling it and convincing people that a symptom checklist and an antidepressant prescription constitutes optimal care.
That is the only way that the current abysmal psychiatric services offered by large health care corporations could get a pass.
George Dawson, MD, DFAPA
References:
1: Rosenbaum L. The Less-Is-More Crusade - Are We Overmedicalizing or Oversimplifying? N Engl J Med. 2017 Dec 14;377(24):2392-2397. doi: 10.1056/NEJMms1713248. PubMed PMID: 29236644.
2: Sullivan K. The rise and decline of the Dartmouth Atlas. The Health Care Blog, September 25, 2016 http://thehealthcareblog.com/blog/2016/09/25/the-rise-and-decline-of-the-dartmouth-atlas/
Monday, December 11, 2017
Takotsubo's and Antidepressants
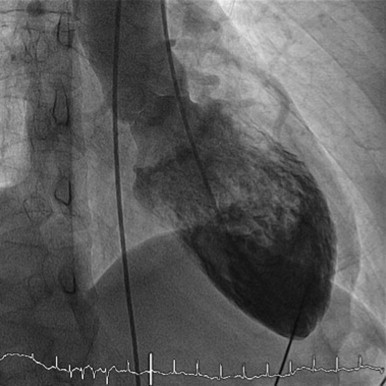
Takotsubo's cardiomyopathy (TC) is a form of acute dilated cardiomyopathy. It was first described in Japan in 1990 and since then there have been increasing reports of the problem. It is an uncommon problem thought to be due to excessive catecholamines and their effects on heart muscle. A definitive source on the autonomic nervous system points out that the plasma levels of catecholamines are extreme and consistent with activation of both the sympathetic nervous system and adrenal medulla (15). As seen in the above ventriculography the left ventricle is dilated and elongated. The typical presentation is acute coronary syndrome (dyspnea, chest pain, syncope) with a low to modest levels of troponin - a biomarker for cardiac tissue damage. Electrocardiogram show T wave inversions and ST segment elevation. Echocardiograms show The imaging results generally show and acutely dilated and lengthened left ventricle and wall motion abnormalities. Overall this pathology represents a low number of cases with acute coronary syndrome. Most people recover in up to 4 weeks if the diagnosis is made and the patient is treated. That treatment may involve the discontinuation or changing of antidepressant medications.
One of the questions that psychiatrists face is whether to resume existing therapy or withhold in the case of a new diagnosis of cardiac disease. It is not an easy decision. At least it is not a straightforward as it used to be. In complicated situations in the past, psychiatric medications were routinely discontinued. Today - talking with a consultant it is much more common to hear: "Yes those adverse reactions are possible but if the patient needs the medication - they need the medication." There are no strict rules on the issue like there seem to have been at one point. The ideal medication for cardiac problems in psychiatry has limited effect on cardiac conduction and is benign in terms of hemodynamics. Those effects are difficult to predict on both an individual and group basis in that small number of cases where the response is more critical. As an example, I contacted an expert on hypertension and he advised me that he was generally unconcerned about the two antidepressants that I end up monitoring for possible effects - buproprion and venlafaxine. In his experience, he though they added an average of 3 mm Hg systolic hypertension and that is trivial. I am seeing people on more medications who may have alcohol and substance use problems that can significantly compound the hypertension problem. The blood pressure I see are considerably higher in some cases where these antidepressants are used or added.
TC was first reported in 1990. Case reports on TC and the association with antidepressants began to show up in about 2008 with a case report involving nortriptyline (15). A recent letter to the editor (1) points out the trend and the possible correlation based on the temporal relationship between antidepressant initiation/titration and TC (4). In that paper the authors identified 8 cases in the literature and a case series of 6 patients. They report their case as well as 8 additional cases and the demographic, clinical, laboratory data of all of the patients. Eight of 9 cases were women in the age range of 37 to 82. In each of these cases the patient was taking an SNRI (venlafaxine, desmethylvenlafaxine, and duloxetine). The youngest patient overdosed on 2100 mg venlafaxine. The presumed etiology in the initial cases was a stressor that lead to catecholamine excess and cardiomyocyte toxicity, but in 5/9 cases a stressor was not present. Although the authors note the association with SNRI type antidepressant case have been reported with atomoxetine (an NRI), fluoxetine (an SSRI), with a case of serotonin syndrome, and withdrawal of antidepressant therapy. The association in the case reports may be seem more robust that it really is. Although the case reprts and incident of ACS are low - the sheer number of cases (one every 25 seconds in the USA) assures that even low prevalence disorders like TC will occur in significant numbers.
What are the implications for psychiatrists? The prototype for me in this case was clozapine. Clozapine is an atypical antipsychotic with many side effects. The focus for most psychiatrists has been the neurophil monitoring and prevention of agranulocytosis. Clozapine also has significant cardiac side effects including acute myocarditis with typical symptoms of myocarditis. I think that anyone prescribing clozapine needs to be aware of these symptoms and monitor the patient for them. In this case the cardiac review of systems is critical along with the physical examination of the patient at every visit or at least until they are on a stable dose of medication.
Antidepressants are generally approached in a more casual manner than antipsychotics. Every patient needs to be carefully screened for side effects and the medication needs to be stopped or modified as indicated. There should be more vigilance after FDA warnings about the QTc prolonging properties of antidepressants specifically citalopram. But if a patient has an arrhythmia questions on a review of systems are probably not enough. I can say that because I take my own vitals signs and know that patients with new onset arrhythmias (atrial fibrillation, ventricular premature contractions, bigeminy, etc) are generally missed with standard blood pressure measurement systems and the patient is unaware that they are no longer in normal sinus rhythm. In many cases tachycardia and other symptoms of acute coronary syndrome are the only findings. These case reports illustrate to me that psychiatrists and primary care physicians prescribing these medications need to have a low threshold for testing and referral both to the emergency department and cardiology. As previously posted - if you are in a large mental health clinic and have enough support staff - consider getting an ECG machine. A faxed abnormal ECG and a verbal report is a sure way to get the attention of a cardiologist or emergency medicine physician.
In terms of the eventual epidemiology and pathophysiology of TC, inferring that SNRIs are definitely involved in either is highly speculative at this point. In many ways the situation resembles the problem of seeing Vitamin D deficiency as being causative for any number of disorders when there is a high prevalence of Vitamin D deficiency in the population. I have always seen SNRIs as better tolerated than SSRIs with a cardiac safety profile that might be slightly better if the prolonged QTc interval issue with citalopram was real. The best way to study the issue is to identify large numbers of patients with TC on echocardiogram and compare them to a control group and see if the correlation between SNRIs and TC is real. As far as I know that study has not been done.
In the meantime, pay close attention to the cardiovascular status of patients on antidepressants, especially during the titration phase and if they may have a high catecholamine load inferred from clinical anxiety, exacerbations of physical illness, and stress levels. . Use findings like tachycardia as a prompt for more focused questions and examination.
Takotsubo's cardiomyopathy is just another medical condition that can complicate psychiatric treatment - that you do not want to miss.
George Dawson, MD, DFAPA
References:
1: Madias JE. Venlafaxine and takotsubo syndrome: Can we learn more from published patient cases? Int J Cardiol. 2016 Dec 15;225:73-74. doi: 10.1016/j.ijcard.2016.09.133. Epub 2016 Oct 1. PubMed PMID: 27716552.
2: Conrad SK, Catalano MC, Catalano G. The Use of Fluoxetine in a Patient With
Takotsubo Cardiomyopathy. J Psychiatr Pract. 2016 May;22(3):234-8. doi:
10.1097/PRA.0000000000000151. PubMed PMID: 27123803.
3: Naguy A, Al-Mutairi H, Al-Tajali A. Atomoxetine-related Takotsubo
Cardiomyopathy. J Psychiatr Pract. 2016 May;22(3):232-3. doi:
10.1097/PRA.0000000000000152. PubMed PMID: 27123802.
4: Madias JE. Withdrawal of prolonged antidepressant therapy and Takotsubo
syndrome. Heart Lung. 2014 Nov-Dec;43(6):578. doi: 10.1016/j.hrtlng.2014.06.053.
Epub 2014 Jul 22. PubMed PMID: 25063669.
5: Dias A, Franco E, Figueredo VM, Hebert K, Quevedo HC. Occurrence of Takotsubo
cardiomyopathy and use of antidepressants. Int J Cardiol. 2014 Jun
15;174(2):433-6. doi: 10.1016/j.ijcard.2014.04.028. Epub 2014 Apr 13. PubMed
PMID: 24768456.
6: Kitami M, Oizumi H, Kish SJ, Furukawa Y. Takotsubo cardiomyopathy associated
with lithium intoxication in bipolar disorder: a case report. J Clin
Psychopharmacol. 2014 Jun;34(3):410-1. doi: 10.1097/JCP.0b013e3182a95a27. PubMed
PMID: 24699038.
7: Marabotti C, Venturini E, Marabotti A, Pingitore A. Delayed multifocal
recurrent stress-induced cardiomyopathy after antidepressants withdrawal. Heart
Lung. 2014 May-Jun;43(3):225-30. doi: 10.1016/j.hrtlng.2014.03.003. PubMed PMID:
24794783.
8: Neil CJ, Chong CR, Nguyen TH, Horowitz JD. Occurrence of Tako-Tsubo
cardiomyopathy in association with ingestion of serotonin/noradrenaline reuptake
inhibitors. Heart Lung Circ. 2012 Apr;21(4):203-5. doi:
10.1016/j.hlc.2011.12.004. Epub 2012 Jan 27. PubMed PMID: 22285074.
9: Rotondi F, Manganelli F, Carbone G, Stanco G. "Tako-tsubo" cardiomyopathy and
duloxetine use. South Med J. 2011 May;104(5):345-7. doi:
10.1097/SMJ.0b013e318213f3e5. PubMed PMID: 21606714.
10: Trohman RG, Madias C. Duloxetine-induced tako-tsubo cardiomyopathy:
implications for preventing a broken heart. South Med J. 2011 May;104(5):303-4.
doi: 10.1097/SMJ.0b013e318213d10f. PubMed PMID: 21606702.
11: Forman MB, Sutej PG, Jackson EK. Hypertension, tachycardia, and reversible
cardiomyopathy temporally associated with milnacipran use. Tex Heart Inst J.
2011;38(6):714-8. PubMed PMID: 22199446; PubMed Central PMCID: PMC3233339.
12: Selke KJ, Dhar G, Cohn JM. Takotsubo cardiomyopathy associated with titration
of duloxetine. Tex Heart Inst J. 2011;38(5):573-6. PubMed PMID: 22163139; PubMed
Central PMCID: PMC3231522.
13: Mehta NK, Aurigemma G, Rafeq Z, Starobin O. Reverse takotsubo cardiomyopathy:
after an episode of serotonin syndrome. Tex Heart Inst J. 2011;38(5):568-72.
PubMed PMID: 22163138; PubMed Central PMCID: PMC3231548.
14: Christoph M, Ebner B, Stolte D, Ibrahim K, Kolschmann S, Strasser RH, Schön
S. Broken heart syndrome: Tako Tsubo cardiomyopathy associated with an overdose
of the serotonin-norepinephrine reuptake inhibitor Venlafaxine. Eur
Neuropsychopharmacol. 2010 Aug;20(8):594-7. doi: 10.1016/j.euroneuro.2010.03.009.
Epub 2010 May 7. PubMed PMID: 20451358.
15: De Roock S, Beauloye C, De Bauwer I, Vancraynest D, Gurne O, Gerber B,
Hantson P. Tako-tsubo syndrome following nortriptyline overdose. Clin Toxicol
(Phila). 2008 Jun;46(5):475-8. doi: 10.1080/15563650701519786. PubMed PMID:
18568805.
16: Wachira JK, Stys TP. Cardiovascular disease and bridging the diagnostic gap. SD Med. 2013 Sep;66(9):366-9. Review. PubMed PMID: 24279112.
17: Goldstein DS. Catecholamines 101. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2010;20(6):331-352. doi:10.1007/s10286-010-0065-7.
Attribution:
"Levocardiography in the right anterior oblique position shows the picture of an octopus pot, which is characteristic for Takotsubo cardiomyopathy."
Hammer N, Kühne C, Meixensberger J, Hänsel B, Winkler D. Takotsubo cardiomyopathy - An unexpected complication in spine surgery. Int J Surg Case Rep (2014). Link Used per open access license.
17: Goldstein DS. Catecholamines 101. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2010;20(6):331-352. doi:10.1007/s10286-010-0065-7.
Attribution:
"Levocardiography in the right anterior oblique position shows the picture of an octopus pot, which is characteristic for Takotsubo cardiomyopathy."
Hammer N, Kühne C, Meixensberger J, Hänsel B, Winkler D. Takotsubo cardiomyopathy - An unexpected complication in spine surgery. Int J Surg Case Rep (2014). Link Used per open access license.
Subscribe to:
Posts (Atom)