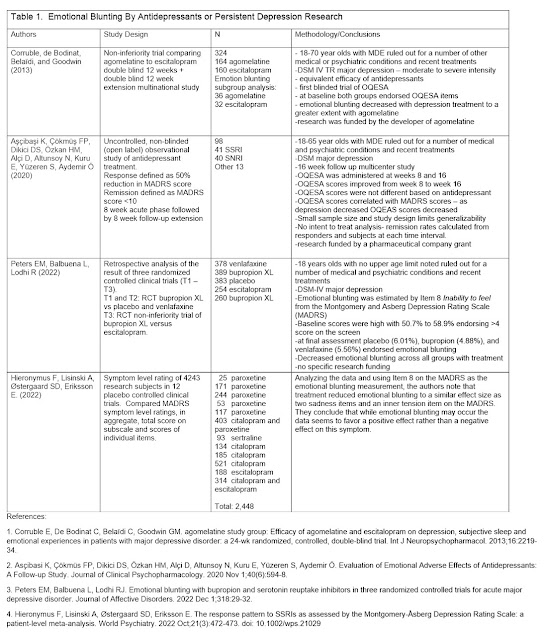
Ron Pies and I reviewed three recent papers on emotional blunting in this paper called Antidepressants Do Not Work by Numbing Emotions. It is a very self-explanatory essay that I encourage anyone to read if they have an interest in that topic specifically or in the general repetitive criticism that only our field seems to enjoy. I have previously commented on the rhetorical aspects to a previous paper and recent publications allow us to address specific scientific issues. The main argument that emotional blunting is the mechanism by which antidepressants work - has no scientific merit as explained in the essay. The basic argument is that if emotional blunting is rated at baseline before any antidepressants are started it is present and as treatment begins and starts to work – emotional blunting decreases as the depression remits. That led the authors we reviewed to conclude it was more likely a symptom of depression than either a mechanism of action or a side effect.
1: Measurement – is a better measurement needed?
In the reviewed studies we made some specific comments on
the methodologies used to detect emotional blunting specifically Item 8 of the
Montgomery Åsberg Depression Rating Scale (MADRS) or the Oxford Depression
Questionnaire (ODQ). The ODQ was previously the Oxford Questionnaire on the
Emotional Side-effects of Antidepressants (OQESA or OQuESA).
The single item on the MADRS provides the most unbiased
assessment of emotional blunting and is a single question worded very much like
a clinician might ask to assess the problem. The ODQ has more questions and a
specific question where the subject is asked to estimate whether or not the
antidepressant is contributing to antidepressant side effects. Since no other potential etiologies (like
depression) were considered we thought that these were questions that
might lead to predictable biases like choosing antidepressants as the cause of
decreased emotional range rather than the depression.
Are there better questionnaire designs to eliminate bias and allow for quantification. Two good examples include the Attributional Style Questionnaire (ASQ) (1) and the Cognitive Style Questionnaire (CSQ) (2). In both of these questionnaires - subjects are asked in an open format to write down what they consider to be the cause of a hypothetical situation, answer a question about that situation, and then rate the important of the cause. The questions are all focused on perceived internal and external causes of depression consistent with the cognitive theory of depression. There is no reason why a similar questionnaire could not be designed to find cognitive and emotional side effects of medications. It could be validated by including questions about the known physical side effects of medication.
2: Normal subjects taking antidepressants:
In thinking about unbiased opinions about the emotional effects
of antidepressants my mind wandered back to a paper I read in the American
Journal of Psychiatry many years ago.
It was easy to remember because it involved giving fluoxetine to research
subjects who had no known psychiatric diagnoses. In that study – 15 subjects
were enrolled and took placebo for two weeks followed by fluoxetine 10 mg x 1
week then fluoxetine 20 mg/day x 5 weeks and then placebo daily for two weeks. There were assessed weekly on standard scales
for anxiety and depression. They were
also assessed weekly on the General Well Being
Schedule and the Quality
of Life Enjoyment and Satisfaction Questionnaire. Subjects were also assessed weekly for side effects
and only three of the 15 subjects reported side effects and they were nausea,
dyspepsia, and dizziness – all typical SSRI side effects. The authors conclude:
“No significant effects attributable to
fluoxetine were observed on any of the psychological variables examined.
Minimal adverse effects were reported. … Significant mood elevating and other
psychological effects of fluoxetine would appear to be induced only when symptomatic
targets exist.”
Interestingly these authors contrast their work with that
of Peter Kramer (4) and suggest that: “….mood-enhancing and other psychoactive
effects of SSRIs are not a general property of these agents but are manifest in
the context of target symptoms.” A
similar argument has been made about emotional blunting and why it may not
occur in normal subjects.
In a second study of fluoxetine and dothiepin in subjects without
mental illness a 5 week placebo controlled, double blind crossover study was
used (4). Each subject received each
treatment and placebo. The active
treatment lasted for 35 days. Subjects received fluoxetine 20 mg/day or dothiepin
(a tricyclic antidepressant) titrated to 150 mg/day. They were tested at day 10
and day 36. Eleven mood ratings using an
analogue scale were done three times a day. The subjects were also asked is
they had any “problems with your health”. Some increased irritability (3 subjects),
anxiety (one subject), and “mood lowering” (2 subjects) was noted. There were no reports of emotional blunting.
The researchers generally reported:
“Throughout the study period, all subjects
remained well, including during the drug-free periods between treatments.”
In looking for a third study, I found one by David Healy –
a well-known pharmaceutical business critic (6).
This study involved 20 subjects randomized to receive 2 weeks of either
reboxetine or sertraline in a cross over design. There was an option to increase either drug
to the next expected level on day 5 of active drug treatment. Three scales were done on a daily basis
including the Profile Of Mood States (POMS), Positive and Negative Affect Scale
(PANAS), and the Social Adaptation Self Evaluation Scale (SASS). A side effects questionnaire was administered.
Subjects were also asked if they could distinguish the “behavioral effects” of
the drugs and to rate their preferences on an 11 point Likert scale ranging from
“worse than normal (-5) to better than normal (+5). A relevant excerpt from this paper:
“In focus group settings, while still under the blind, half
of the subjects volunteered that sertraline made them mellow, or less
emotionally reactive and that these effects were either appreciated or not, while
yet others described agitation. Effects consistent with a reduction in
emotional reactivity were not described with reboxetine.”
This focus group observation was not observed in any of the
data collected from the mood rating scales.
The authors point out studies suggesting that normal volunteers do not tolerate
medications well and suggest that their study shows that tolerance of the antidepressant
may depend on whether it is a preferred agent of the subject. Side effects listed but not quantitated include
chilblains, sweats, insomnia, nausea, sexual dysfunction, and “jaw or throat dyskinesias
or dystonias on sertraline.”
In summary, my main additional concerns about the emotional
blunting issue and whether it occurs to any extent with antidepressants is one
of accuracy of measurement and why it has been conspicuously absent in clinical
trials until recently – including trials where antidepressants are given to
normal controls (defined as volunteers screened for the presence of any current
or lifetime psychiatric disorders). Although
I suggested a way to get to a much less biased measurement of emotional blunting,
I have a question about whether it can be accurately measured at all in an era
where psychiatric research is often presented as political debate in social
media and the popular press. There have been many examples of how biased press
coverage has misrepresented the effects of psychiatric medications and you only
have to look as far as the Peter Kramer reference and the appendix on “Violence”. A study to look at a better questionnaire to see if this is ever spontaneously mentioned when it is not cued would be useful - but it is probably not any more possible today than asking if the last election was stolen.
On the issue of emotional blunting in clinical practice –
I have seen the equivalent in some patients over the years. I typically discuss
it with people as restricted affective range that either they notice or other
people notice. It typically occurs during reassessments of people who have
partially remitted depressions. At that point in time, it makes sense to discuss
the time course of that phenomenon and try to determine the time course and whether
it is improving or getting worse. After making it explicit at that point – the options
in clinical care include continued observation or an immediate change to a
different medication. That aspect of clinical care would be an interesting
study in itself – because in my experience the majority of people do not want
to change the medication at that initial discussion. I think it is also an element of ongoing informed consent at that point.
At a practical level, I think that the study by Peters,
Balbuena, and Lodhi (7) that we referenced has paved the way for many more
replication studies for any RCTs of antidepressants that used the Montgomery-Åsberg
Depression Rating Scale (MADRS) and that is a very significant number.
It also requires a degree of biological sophistication to
realize that brain systems are so complex and individualized that you cannot
expect a medication to affect everyone in the exact same way – either positively
or negatively. In fact, you cannot have that expectation in attempting to treat
far less complex organ systems. Responses to treatment and side effects in medicine are always probability statements.
George Dawson, MD, DFAPA
References:
1. Peterson C, Semmel A, Von Baeyer C, Abramson LY,
Metalsky GI, Seligman ME. The attributional style questionnaire. Cognitive
therapy and research. 1982 Sep;6(3):287-99.
2. Haeffel GJ, Gibb
BE, Metalsky GI, Alloy LB, Abramson LY, Hankin BL, Joiner Jr TE, Swendsen JD.
Measuring cognitive vulnerability to depression: Development and validation of
the cognitive style questionnaire. Clinical Psychology Review. 2008 Jun
1;28(5):824-36
3. Gelfin Y, Gorfine
M, Lerer B. Effect of clinical doses of fluoxetine on psychological variables
in healthy volunteers. Am J Psychiatry. 1998 Feb;155(2):290-2. doi:
10.1176/ajp.155.2.290. PMID: 9464215.
4. Kramer PD: Listening to Prozac. New York, Penguin, 1993
5. Wilson SJ, Bailey
JE, Alford C, Weinstein A, Nutt DJ. Effects of 5 weeks of administration of
fluoxetine and dothiepin in normal volunteers on sleep, daytime sedation,
psychomotor performance and mood. J Psychopharmacol. 2002 Dec;16(4):321-31.
doi: 10.1177/026988110201600406. PMID: 12503831.
6. Tranter R, Healy
H, Cattell D, Healy D. Functional effects of agents differentially selective to
noradrenergic or serotonergic systems. Psychol Med. 2002 Apr;32(3):517-24. doi:
10.1017/s0033291701005086. PMID: 11989996.
7. Peters EM, Balbuena L, Lodhi RJ. Emotional blunting
with bupropion and serotonin reuptake inhibitors in three randomized controlled
trials for acute major depressive disorder. J Affect Disord. 2022 Dec 1;318:29-32.
doi: 10.1016/j.jad.2022.08.066. Epub 2022 Aug 24. PMID: 36029876.
8. Hieronymus F, Lisinski A, Østergaard SD, Eriksson E. The response pattern to SSRIs as assessed by the Montgomery-Åsberg Depression Rating Scale: a patient-level meta-analysis. World Psychiatry. 2022 Oct;21(3):472-473. doi: 10.1002/wps.21029. PMID: 36073711; PMCID: PMC9453909.
Supplementary:
I was made aware of a new analysis of MADRS data (8) today after writing the above essay. Those authors analyzed MADRS ratings from 4,243 subjects participating in twelve acute phase placebo‐controlled trials of an SSRI in major depression and looked at emotional blunting on Item 8 of the scale. They found that treatment reduced emotional blunting with the same effect size as other items on the scale. They agree with the opinion that emotional blunting should not be ignored but in the case of these antidepressant trials the ratings moved in a favorable direction.
Updated Table including this new analysis (click to see a clearer version):
Graphic Credit:
Nasa surface of Europa per their media guidelines