The Gateway Drug hypothesis proposes that using a particular drug increases the likelihood that there will be a progressions to using other drugs of abuse. The competing hypothesis is that there is a general predisposition to using more than one drug in people susceptible to addiction and that it does not depend on any sequence of exposures. In this Shattuck Lecture in the New England Journal of Medicine, Eric and Denise Kandel examine the epidemiology and molecular biology of nicotine use and the development of addictions. Most addiction treatment centers recognize the importance of nicotine cessation in improving abstinence rates from the primary drugs of choice. Most of that depends on series of cases discharged from treatment centers. The idea of nicotine also has importance because one of the approaches to nicotine cessation depends on nicotine substitution and it is important to know if that treatment intervention might lead to increased risk for ongoing substance use problems. It also has implications for electronic cigarettes (e-cigarettes). There is a general idea that any form of nicotine that does not involve exposure to combustible or chewed tobacco components is preferred because of all of the conditions associated with the physical and combustible products of tobacco. If nicotine alone places one at risk for using another drug like cocaine, the risk/benefit decision will need to be reconsidered.
The authors briefly reviewed the epidemiology showing of all US adults who had ever used cocaine, the vast majority of them (87.9%) smoked cigarettes before using cocaine. Only 3.5% used cocaine first. The rate of cocaine dependence was highest (20.2%) among those who smoked cigarettes before using cocaine. They proceed to use an animal model to examine the possible priming effects of nicotine and to possible elucidate the mechanism. The animal models of addiction in mice included locomotor sensitization and conditioned place preference. In both of these models, micd primed with nicotine first and then treated with nicotine and cocaine showed the expected addicted response with heightened locomotor sensitization and place preference. Mice primed with cocaine did not.
They proceed to look at the effect on synaptic plasticity and the model used was long term potentiation (LTP) in the nucleus accumbens (NAcc). The predominate neurons in the NAcc are medium spiny neurons (MSNs) and they are innervated by dopaminergic neurons projecting from the ventral tegmental area (VTA) and glutamatergic neurons from the prefrontal cortex and the amygdala. Reducing excitatory (glutamatergic) input to the NAcc reduces inhibitory output to the VTA leading to more dopaminergic input to the NAcc or greater reward. Repeated cocaine administration leads to reduced LTP in the excitatory synapses of the NAcc. A single injection of cocaine in a mouse primed with 7 days of nicotine exposure leads to marked reduction in LTP. Nicotine alone, or nicotine after cocaine had no effect on LTP. Priming with nicotine causes a change in neuronal plasticity that increases cocaine associated reward.
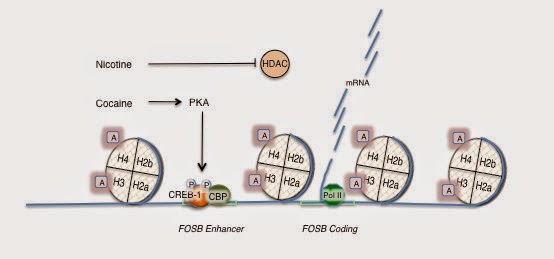
The authors turned to known gene expression markers of addiction in the striatum specifically the expression of FosB. They demonstrated that nicotine alone for seven days increased FosB expression and adding cocaine led to a further 25% expression. The next step was to examine if nicotine alters the chromatin structure at FosB promotor of the gene and they looked at the acetylation of histones H3 and H4 at the FosB promotor. Nicotine alone increased the acetylation of both histones, cocaine alone increased the acetylation of H4 only. They went on to demonstrate that the acetylation was widespread after nicotine exposure throughout the striatum. Cocaine alone had no similar effect. They went on to clarify that increase acetylation was due to inactivation of histone deacetylase activity (HDAC) and not activation of acetylases. They carried out additional pharmacological studies to confirm that the hypoacetylated state (caused by nicotine) leads to depression of LTP associated with cocaine and that further it cannot be rapidly reversed. The authors investigated if nicotine enhanced cocaine induced LTP in the amygdala and hippocampus and found that it did.
This fairly intensive research program and series of experiments allowed the authors to conclude that nicotine has a unidirectional priming effect and it works through an acetylation mechanism by affecting HDAC activity. The mechanism explains what is seen in human populations at the epidemiological level and in mice at the experimental level. That has obvious implications for treatment as well as drug development. It also points out that e-cigarettes are potentially as much of a potential gateway drug as combustible cigarettes. In clinical practice it is also fairly common to see patients with addiction who are continuing to use nicotine substitutes (gum, lozenge, patch) long after they have stopped smoking. If the mechanism elucidated by Kandel and Kandel is accurate it will be important to discuss the implications of continued nicotine exposure.
Kandel's work is always compelling because of his broad view of science and psychiatry. He is as comfortable discussing psychoanalysis and Freud as he is talking about molecular biology. In this case he combines views on epidemiology and molecular biology in a very compelling story and suggests it might be a broader model for how gateway drugs work. As he is drawing his conclusions there is still room for the competing hypothesis and he makes this explicit. His work is always a breath of fresh air compared to the current zeitgeist of political arguments about science and psychiatry often from people who know very little about either subject. Go to the article at the link below and read this paper and compare it to his 1979 article Psychotherapy and the Single Synapse. That covers at least 34 years of studying synaptic plasticity and it is a remarkable accomplishment.
George Dawson, MD, DFAPA
1: Kandel ER, Kandel DB. Shattuck Lecture. A molecular basis for nicotine as agateway drug. N Engl J Med. 2014 Sep 4;371(10):932-43. doi: 10.1056/NEJMsa1405092. PubMed PMID: 25184865. (free full text).
Supplementary 1: Drawing depicts nicotine inhibiting HDAC leading to increased acetylation of histones per the above discussion. CREB-1 = cyclic AMP response-element-binding protein; CBP = CREB-binding protein (acetylates histone H4); PKA= protein kinase A; A=acetyl groups, P=phosphate groups; H2a, H2b, H3, H4 = histone proteins in chromatin; Pol II = RNA polymerase II (catalyzes synthesis of DNA to mRNA).